The Ministry of Employment and Labor (MoEL) of South Korea revised and issued the Occupational Safety and Health Act (OSHA) in 2019. The revised South Korean OSHA regulations has come into force since January 16th 2021. The revised regulation applies to manufacturers and importers of hazardous chemicals based in Korea. Related enterprises should submit the (M)SDS to MoEL before manufacturing or importing.
1. Scope of Exemption:
- Single substance or mixture which has no classification
- Article in solid form that do not pose a risk of workers' exposure to a product or specific chemical substance contained in the product during handling (However, except products that contain substances requiring special handling control)
- Health functional food / Agricultural Pesticides / Nacrotics and psychotropic / Fertilizer / Feed / Foods and food additives / Medicines and quasi-drugs / Radioactive substances / Hygiene / Medical devices / Advanced biopharmaceuticals / Waste / Cosmetics
- Explosives under Article 2 (3) of the 「Act on the Safety Management of Guns, Swords, and Explosives, etc.」
- Among the household chemical products and biocidal products subject to safety confirmation pursuant to subparagraphs 4 and 8 of Article 3 of K-BPR, products provided for the daily use of general consumers;
- Raw materials under subparagraph 2 of Article 2 of the 「Ambient Radiation Safety Management Act」
2. Key updates on SDS standards (MoEL No.2020-130):
- CBI application restrictions, the following substances cannot be applied:
- prohibited substances, substances requiring permission, hazardous substances subject to management, harmful factors subject to measurement of the work environment, hazardous factors subject to a special health examination and substances having a physical hazard, health hazard or environmental hazard under K-REACH
- At least one out of 48 categories of use to choose from when submitting the SDS via the portal
- The concentration for substitute chemicals is required as a range, whereby, if the concentration is below 25%, the range can be +/- 10% and if the concentration is equal or above 25%, the range can be +/- 20%.
- Classification and terminology updates:
- New gas category: pyrophoric gases
- Some H/P term updates
- Added 1A/B/C category for skin corrosion, added 2A/2B category for eye irritation, and added 1A/B category for sensitization
3. Korea Label Standards (MoEL Notice No.2020-130)
- Language should be written in Korean
- Include 4 pictograms & 6 precautionary statements
- Must contain the contact info of Korean legal entity
- If the capacity is less than 100ml, simplified label can be used
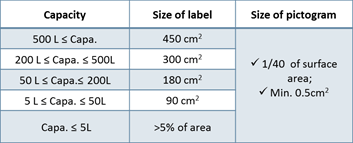
Table 1. Capacity and Size of label requirement
KOSHA SDS submission
From January 16th 2021, for manufacturers and importers of hazardous chemicals based in Korea, they should submit the (M)SDS to MoEL before manufacturing or importing in accordance with current amended regulation. However, there is a transitional period for enterprises that previously prepared an SDS in accordance with Article 41(1) or (6) of the former K-OSHA Act. Those meeting the exception may submit the SDS to MoEL within specified deadlines based on manufacture or importation volumes as noted below:
- ≥ 1000tpa, before 16 Jan. 2022;
- 100-1000tpa, before 16 Jan. 2023;
- 10-100tpa, before 16 Jan. 2024;
- 1-10tpa, before 16 Jan. 2025;
- Less than 1tpa, before 16 Jan. 2026;
Manufacturers of hazardous chemicals based outside of South Korea may entrust an Only Representative (OR) based in Korea to submit the (M)SDS. (M)SDS shall be submitted through the official online IT system as shown below.

Fig 1. Submission Portal for MSDS
There are three options to submit (M)SDS with required materials:
-
Submit (M)SDS specifying 100% composition information (no matter the chemical ingredients are hazardous or non-hazardous);
-
Only submit (M)SDS specifying hazardous ingredients and a separate document providing information of other ingredients will also be submitted to MoEL together with the (M)SDS; and
-
Only submit (M)SDS specifying hazardous ingredients and a statement signed by foreign companies saying that all the non-disclosed ingredients are non-hazardous substances will also be submitted to MoEL along with the (M)SDS. This option is for imported products only.
For overseas manufacturers, confirmation of chemical substance with LoC should also be submitted with (M)SDS together through online IT system.
After submitting the (M)SDS, related enterprises will be granted a serial number (AA00000-0000000000)
issued by MoEL. The number shall be written on the upper side of the front page on the (M)SDS. If information in the (M)SDS is altered, then related enterprises must submit the updated (M)SDS to MoEL as soon as possible.
In principle, manufacturers of non-hazardous ingredients based outside of South Korea do not need to provide (M)SDS. They can appoint an OR based in South Korea to submit full ingredients list instead of the (M)SDS.
CBI Application
For hazardous chemicals exceeding the concentration limit, they must be reflected in the MSDS. However, manufacturers or importers are allowed for applying for CBI protection and to use an alternative name and/or alternative content for claiming trade secrets, but they need to get approval from MoE first. It is worth mentioning that enterprises cannot apply for CBI Protection for substances that are prohibited in South Korea, restricted substances, hazardous substances under control, CMR substances, etc.
Information required for the CBI Protection Application includes:
-
MSDS
-
Applicant information;
-
Reason for CBI Protection;
-
Hazardous classification of the chemical products and the ingredients
-
Other documents
After the application for CBI Protection is approved, MoEL will issue an authorization number to the applicant. Applicant shall fill it in Part 3 of the (M)SDS. The validity period for CBI Protection is 5 years and enterprises may apply for extension of the validity period. Substances that have registered under K-REACH and passed the CBI assessment could not be used under CBI Protection under OSHA.
Our Service
-
CIRS Korea only representative services
-
Korea MSDS preparation/update/review/submission service
-
CBI application related services
-
Regulation training service