On November 30, 2022, the European Commission issued Regulation (EU) 2022/2340 amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council, which included green tea extracts containing (-)- epigallocatechin-3-gallate (EGCG) in restricted substance list. The Regulation has been in force since December 21, 2022, and food items that include green tea extracts containing EGCG, which do not comply with the requirements of this Regulation and were lawfully placed on the market before the entry into force of this Regulation will no longer be allowed remaining on the market from June 21, 2023.
1. What is (-)- epigallocatechin-3-gallate (EGCG)?
Unfermented green tea results in the presence of flavanols, commonly known as catechins, the most relevant of which is EGCG. At present, catechin has been used as a common ingredient in dietary supplements in America and European countries. EGCG keeps attracting a great number of manufacturers due to its function as an anti-inflammatory and antioxidant and regulating blood lipids.
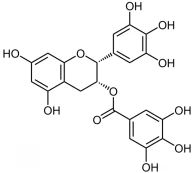
(Chemical structure formula of EGCG)
2, Why is green tea extract containing EGCG included in the list of restricted substances?
In 2015, a potential risk to consumers was associated with the intake of catechins (in particular EGCG in green tea extracts used in the manufacture of food). Norway, Sweden, and Denmark sent a request to the Commission to include green tea extracts containing EGCG in Annex Ⅲ B, which lists the substances whose use in foods is prohibited, restricted, or under Union scrutiny. The Commission, therefore, requested the European Food Safety Authority (EFSA) to deliver a scientific opinion on the evaluation of the safety of green tea catechins.
In March 2018, EFSA adopted a scientific opinion on the safety of green tea catechins and concluded that intake of doses equal to or above 800 mg EGCG per day in the form of a food supplement, was shown to induce a statistically significant increase of serum transaminases in treated subjects compared to control subjects, which is indicative of liver injury. And there is still a possibility of harmful effects on health associated with a daily intake level of less than 800 mg of EGCG from green tea extracts.
However, considering that there were several uncertainties regarding exposure to green tea catechins and their biological and toxicological effects, EFSA was unable to provide advice on a dietary intake of green tea catechins. Therefore the European Commission eventually determined to include green tea extracts containing EGCG in Annex Ⅲ.
Annex III to Regulation (EC) No 1925/2006 is amended as follows:
(1) in Part B, the following entry is inserted in the table, in alphabetical order:
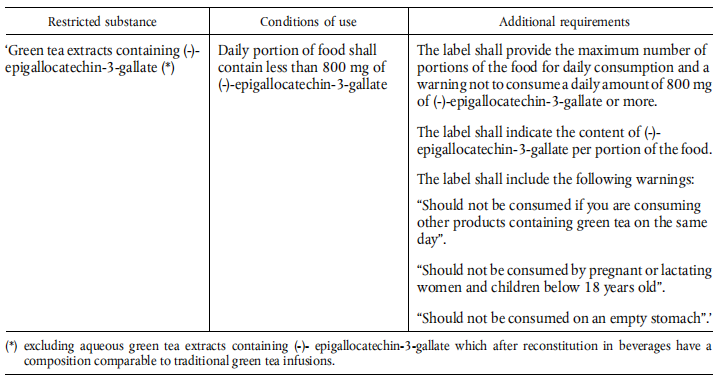
(2) in Part C, the following entry is inserted, in alphabetical order:
Green tea extracts containing (-)- epigallocatechin-3-gallate (*)
(*) excluding aqueous green tea extracts containing (-)- epigallocatechin-3-gallate which after reconstitution in beverages have a composition comparable to traditional green tea infusions.
3. Summary
Seeing that the Regulation entered into force on December 21, 2022, enterprises shall make adjustments to relevant products as well as the labels and warnings attached.
Furthermore, this Regulation should not affect the use in fortified foods and food supplements of (-)-epigallocatechin-3-gallate as a highly purified extract from the leaves of green tea (Camellia sinensis (L.) Kuntze) containing a minimum of 90 % (-) -epigallocatechin-3-gallate.
If you need any assistance or have any questions, please get in touch with us via service@jianzaoshiwang.cn.

