With the aim of maintaining the health food market order, former CFDA carries out supervision of health food by casual inspection, problem-addressing action, case investigation and disposal, and so on. Meanwhile, former CFDA released a series of regulations to strengthen the supervision of health food. Taking the casual inspection as an example, in 2018, the regulations pertaining to food casual inspection are as following:
Main Supervision Regulations of Health Food | Implement Time |
The Proposal on Specific Casual Inspection Work of Food and Health Food | 2018.04.02 |
Implementation Rules on Food Safety Supervision and Casual Inspection (2018 version) | 2018.01.24 |
Plan on Food Safety Casual Inspection of 2018 | 2018.01.08 |
…… | |
In addition, in order to facilitate the product compliance and help companies realize the current supervision of Chinese health food, CIRS collected and analyzed the information of casual inspection results published by former CFDA, then found out that there were four main reasons including adding illegal substances causing health food disqualification. At the same time, statistical analysis was carried out on Complaints and Case investigation and disposal of food (including health food) by CIRS which could help companies have a fuller understanding of supervision on health food and punishment of food safety violations.
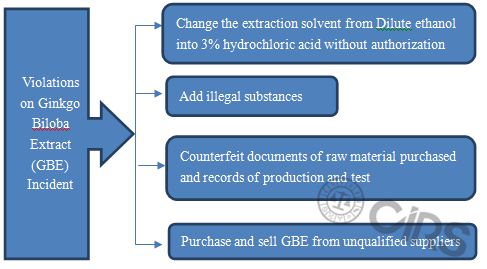
Finally, CIRS analyzed the typical food safety incidents according to some relevant Notices. Taking ginkgo biloba extract as an example, initially the violations were discovered by flight inspection of former CFDA (the detailed violations are shown in the figure below). Subsequently, former CFDA carried out problem-addressing action on “ginkgo biloba extract and its drug” through flight inspection, casual inspection, company self-test and so on. Former CFDA investigated and dealed with violations according to law, they shut down involved companies and recalled ginkgo biloba extract related products.
Section 5 of
- Health Food Supervision Regulations
- The Unqualified Reason of Health food
- Health Food Complaints Report
- The Investigation and Disposal Data of Health Food Cases
- Analysis of Typical Food Safety Incidents
The summary of each section in the Guideline is introduced by a series of articles which are available as following. If it is of your interest, please pay attention to our updates.
Section 1: Information to Know before Health Food Exportation to China
Section 2: How Many Health Food are Registered in China?
Section 3: Chinese Health Food Regulation in Present and Future
Section 4: Timeline and Budget for Health Food Access to Chinese Market
Section 5: How does Chinese Government Supervise the Health Food?
Section 6: Cross-Border E-Commerce (CBEC): New Opportunity for Imported Health Food to Enter the Chinese Market
In addition, if you would like to get the
Editor:
CIRS Food Technical Team: Established in 2012, CIRS Food Technical Team has more than 80% masters with degrees of Food Safety or Food Engineering and more than 50% members from oversea renowned universities. Since it was founded, based on the rich experience of regulatory compliance and deep understanding of Chinese food regulations and industry, food specialists have made comprehensive and reasonable solutions for many oversea food companies to complete Chinese food regulatory compliance progress.
If you have any other questions, please contact us at service@jianzaoshiwang.cn.
Related Article:
Cross-Border Acquisition: Chinese-funded Enterprises Frequently Acquire Foreign Health Food Brand
Data Analysis: Why are Health Food Enterprises Keen to Explore E-Commerce Channel?

