As of February 22, 2021, about 150 imported health food have got filing certificates since health food filing system went online officially on May 1, 2017. Subject to the restriction that the dosage forms of filing health food must meet the requirements of the Main Production Process of Health Food Filing and other relevant regulations, only the following dosage forms are currently available for filing health food in China: tablet, hard capsule, soft capsule, oral liquid and granule.
It is well known that health food filing policy is unprecedented in China, which simplifies the procedures of administrative examination, helps to invigorate the market, and reduces the enterprises’ burden. In order to respond to the policy of streamlining administration and decentralization, SAMR shall make more health food applying for filing instead of the complicated registration. On February 20, 2021, SAMR issued Dosage forms and Technical Requirements of Health Food Filing (2021 version) and Available Excipients for Health Food Filing and Their Usage Rules (2021 version) (hereinafter referred to as the “2021 Excipients Directory”), which will be officially implemented on June 1, 2021. The release of the documents means that gelatin candy and powder will be the available dosage forms for filing health food in China since June 1, 2021.
Different from the existing available dosage forms, gelatin candy and powder are food forms. In order to help overseas companies have a more intuitive understanding of Chinese health food filing requirements, this article takes imported health food with the form of gelatin candy as an example, focusing on the filing procedures, required material and certifying documents for filing, as well as other matters needing attention for filing work.
Imported Health Food Filing Process
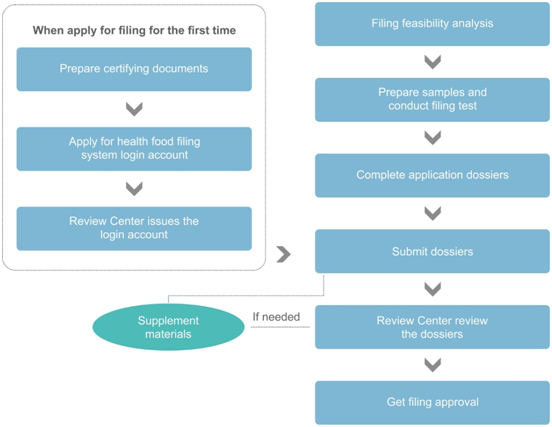
The figure above is a simplified process diagram of the application for the health food filing system login account (hereinafter referred to as the “filing account”) and the application for the product filing. As shown in the figure, before the first filing of imported health food, the applicant needs to obtain the filing account. After that, the applicant shall submit the product filing dossiers online. Usually, when everything goes smoothly, it takes about 8 months from dossier preparation to final receipt of product filing certificate. Among the procedures, the more time-consuming ones are: the issuance of certifying documents, the stability test of the samples, and the official technical reviews.
Generally, in order to shorten the filing period, CIRS Group recommends preparing filing account application and product filing application at the same time. Once the filing account is obtained, the product filing application can be submitted online immediately. In addition, for some specific works, such as sample testing and dossier preparation can also be carried out at the same time, which can further save the time of filing application.
The company filing account application and product filing application first shall be submitted online through the official website (Health Food Filing Management Information System). After the approval by technical review, the paper dossier of the application can be submitted on-site. The first step (online): click on “Get Login Account”, apply for the health food filing account. And the second step (online): log in to the filing system through the obtained login account, and apply for the health food product filing certificate. The homepage of “Health Food Filing Management Information System” is as following:
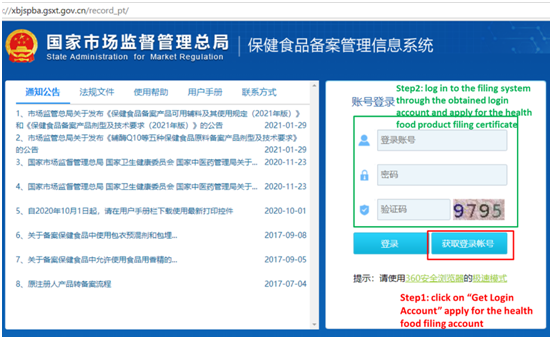
Step 1 online: After clicking “Get Login Account”, enter the following page, fill in the company information and upload relevant certifying documents as required, to apply for a filing account.

Step 2 online: Through the obtained login account, log in to the filing system and enter the following page. Click on "applying for new product filing", and then click on "filing application". Fill in the product information, manufacturer information and domestic application agency information as required, and upload the product information attachments, to apply for the health food filing certificate.
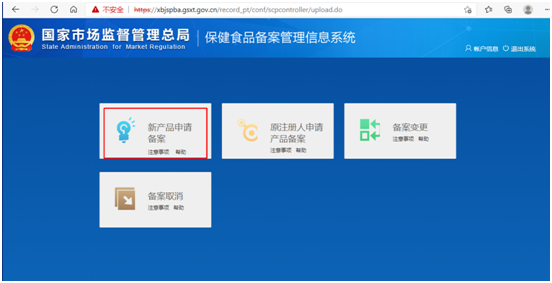
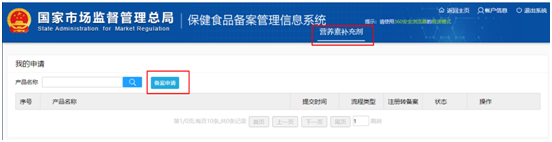
Regardless of whether it is a health food with the dosage form of tablet, hard capsule, soft capsule, oral liquid or granule, or a health food with the form of gelatin candy or powder, the filing process for imported health foods is the same.
In addition to the understanding of health food filing process, the requirements on dossiers of health food filing account application and health food product filing certificate application also require great attention. If the dossier submitted does not meet the requirements, the filing process will be disrupted or even start over. CIRS Group sorted out the required documents based on regulation requirements and practical experience, and listed some points that should be paid attention to in the actual application.
Required Documents for Imported Health Food Filing
First of all, for filing account application, the required documents are as follows:
1. Qualification certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin, proving that the oversea filing applicant is the owner of the health food marketed;
2. Letter of Authorization on the contact of health food filling account application;
3. A scanned copy of the passport of the legal representative of the applicant
Note: The above three documents seem simple, but in fact there are many details that need to be paid attention to. For example, the scanned copy of passport must be clear, complete and in color; if there is a legal representative’s signature in the authorization letter, the signature must be consistent with the passport signature.
Furthermore, for imported health food filing application, what documents shall we prepare?
1. Application form and letter of commitment for authenticity materials (CIRS provide template)
2. Copy of legally registered certificate of the applicant (CIRS provide template)
3. Product formula
4. Product production process material, include producing flow chart and detailed instructions
5. Product safety and health function evaluation materials
5.1 Test report of functional component test, hygiene test and stability test for 3 batches of finished product.
5.2 Explanation on the use of raw materials and excipients, and on the conformity of product label, instruction book, and product technical requirements to relevant Chinese regulations. (CIRS provide template)
6. Type, name and related standards of the package materials that directly contact the product
7. Samples of product label and instruction book
8. Product technical requirements
9. Test report issued by qualified Chinese laboratory, test items shall be all the items listed in the product technical requirements.
9.1 Qualification certifying documents of the laboratory (CIRS provide template)
9.2 Test report including all items listed in the product technical requirements for 3 batches of finished product.
10. Retrieval material related to the product name (CIRS provide template)
11. Other materials proving product safety and health function(s) (CIRS provide template)
12. Certifying documents proving that the product has been marketed more than a year
13. Health food associated standards issued by the product producing country (region) of origin or international organizations
14. Packaging, labels, instruction book for products marketed in the producing country (region) of origin
15. Original power of attorney notarized by local notary organization (CIRS provide template)
16. Certifying document proving GMP compliance of the actual manufacturer who produces the product
17. Self- inspection report to ensure the effective operation of GMP system
It should be noted that, the existing available dosage forms (tablet, hard capsule, soft capsule, oral liquid or granule) for filing health food are all included in the current Chinese Pharmacopoeia, and the technical requirements can refer to the current Chinese Pharmacopoeia and GB 16740. But there is no corresponding national standard for the technical indicators of gelatin candy and powder health food, and available excipients are not exactly the same as the existing dosage forms. Therefore, there are special and additional requirements for health food with the form of gelatin candy or powder, including the technical requirements, available excipients, etc.
The following takes the health food with the form of gelatin candy as an example, to specifically explain the technical requirements and available excipients of this type of product.
The key content in the product instruction of gelatin candy:
The product technical requirement for gelatin candy:
According to the characteristics of gelatin candy, specific requirements have been made on the sensory requirements, physical and chemical indicators, and microbial indicators of them (excluding other regulations related to raw materials and excipients). Taking physical and chemical indicators as an example, the requirements are as follows:
Items | |
Pb (count as Pb), mg/kg | ≤0.5 |
Total As (count as As), mg/kg | ≤0.5 |
Total Hg (count as Hg), mg/kg | ≤0.3 |
Loss on drying, g/100g | Vegetable gelatin type: ≤18.0 Animal gelatin type: ≤20.0 Starch type: ≤18.0 Mixed type: ≤35.0 Sandwich type, coating and coating polishing type: meet the requirements of the main candy Other gelatin types: ≤20.0 |
Reducing sugar (count as glucose), g/100g | ≥10.0 Sandwich type, coating and coating polishing type: meet the requirements of the main candy. Gelatin candy of sugar-free gelatin type does not have this indicator. |
Monosaccharides and disaccharides, g/100g | ≤0.5, only gelatin candy of sugar-free gelatin type has this indicator. |
Main Production Process of gelatin candy:
Melting gel and sugar, boiling, mixing, blending, filtering, inflating, molding, drying, stirring with granulated sugar, coating, polishing, smearing, packaging, etc.
The scope of application of gelatin candy:
At present, products using vitamins and minerals as raw materials that have been included in the Health Food Raw Materials Directory can use this form.
The available excipients for gelatin candy:
In the 2021 Excipients Directory, 197 kinds of excipients are available for filing health food. As gelatin candy is a kind of food form, the available excipients are less than other dosage forms such as capsule, tablet, and granule, and only 151 kinds of excipients can be used for the production of gelatin candy. Excipients such as iron oxide black, iron oxide red, iron oxide yellow, dextrin, silicon dioxide, fumaric acid, monascus yellow pigment, etc., are not allowed for gelatin candy.
Judging from health food filing procedures and the documents mentioned above, in fact, health food filing is not difficult compared with health food registration. It is not necessary for filing to conduct toxicological test and functional test, R&D report is not required as well. However, for imported health food companies, the application of health food filing account and product filing may still face the following difficulties:
- Deep understanding of Chinese regulations: In China, there are lot of rules and regulations related to health food filing, and the content is exactly rich. Only regulatory specialists who are very familiar with the regulations can have a comprehensive understanding and make as few detours as possible.
- Rich practical experience: Besides the regulation requirements, CIRS found that some specific details may not be explained in regulations, but are still required by the review experts of health food filing. Therefore, regulatory specialists who are responsible for the filing affairs shall have the rich experience and communicate with review experts frequently, so as to get the detailed requirements.
- Tracking regulations and policies: Health food regulation is developing, and the review experts are becoming stricter on the submitted dossiers. In CIRS Food Division, we have dedicated personnel to monitor the updating regulations and notify everyone. Meanwhile, when dealing with a new project, if any regulatory specialist finds that the requirements have changed, the one shall notify the others to avoid detours in subsequent projects.
- The contact of the applicant: The contact must have a Chinese mobile phone number and speak Chinese. In addition, the contact person shall have some knowledge on health food regulations and the submitted dossiers as he/she needs to answer the call from the review expert and make some explain in order to reduce the possibility of dossier correction and/or supplement, so that make the project more smoothly.
For Chinese health food companies, it may not be hard to reach the above four requirements. But for oversea companies, it may take a lot of time and money to overcome the difficulties. As a result, almost all imported health food have entrusted domestic agencies to deal with the filing. CIRS Group was established in accordance with the laws of the People's Republic of China, and is qualified to act as an agent for health food filing. Any company has any question or need any help, is welcome to contact us at service@jianzaoshiwang.cn.

