On December 1, 2020, the SAMR officially issued Coenzyme Q10, Melatonin, Fish oil, Broken Ganoderma lucidum spore powder and Spirulina Health Food Raw Materials Directory (hereinafter referred to as “Health Food Raw Materials Directory”). Since then, for health foods that use one of these five substances as single active ingredient: 1) Domestic product applicants should apply for filing after the release of the Health Food Raw Materials Directory, and overseas applicants still need to apply for registration; 2) For domestic and imported registration applications accepted before the release of the Health Food Raw Materials Directory, and health foods that have been registered, an application for filing by “registration transfer to filing” is required.
What is “registration transfer to filing”?
It is a kind of filing application method for registered health foods or products whose registration application has already been accepted.
Why applying for “registration transfer to filing” instead of directly applying for filing as a new product?
Domestic health foods: “registration transfer to filing” is not the only option, but there are favorable policies if you choose it.
For domestic health foods that use one of these five substances as single active ingredient, applicants can either directly apply for filing as new products, or apply for filing by “registration transfer to filing”. The advantage of “registration transfer to filing” is that, some products do not need to conduct functional components or characteristic ingredients tests, hygiene tests and stability tests (this favorable policy is also applicable to imported products), which greatly shortens the application period.
-
When a health food that has obtained the registration certificate is going to transfer to filing, if the name and dosage of raw materials and excipients, technical requirements are not changed, only all item test need to be conducted, while functional components or characteristic ingredients tests, hygiene tests and stability tests are not required;
-
When a health food whose registration application was accepted before the release of the Health Food Raw Materials Directory is going to transfer to filing, if the name and dosage of raw materials and excipients, technical requirements are not changed, the functional components or characteristic ingredients tests, hygiene tests and stability tests report can directly be instead by the test reports submitted during the registration application, and all item test shall be provided as well.
Imported health foods: “registration transfer to filing” is the only option.
The filing scope however, only includes the domestic products in China, and overseas products still need to apply for health food registration.
For such products that have obtained the registration certificate, applicants cannot apply for renewal of registration, but should apply for filing by “registration transfer to filing” during the validity period of the registration certificate.
For imported products whose registration application were accepted before the release of the Health Food Raw Materials Directory, overseas applicants should also apply for filing by “registration transfer to filing”.
“Registration transfer to filing” involves two steps: 1) Confirm that the applicant is an eligible registration applicant by SAMR. 2) Applicants apply for product filing. Specific information is as follows:
1. Confirming the applicant is an eligible registration applicant by SAMR (hereinafter referred to as “confirmation of the qualification of original registrant”).
Which applicants are allowed to apply for filing by “registration transfer to filing”?
As mentioned above, applicants whose products using any of the five substances as single active ingredient have been registered or registration applications were accepted before the release of the Health Food Raw Materials Directory, and the products comply with relevant technical requirements for filing, are allowed to apply for filing by “registration transfer to filing”. If some raw materials or their dosages cannot meet Health Food Raw Materials Directory and the technical requirements for filing, but the applicant agrees to adjust product raw materials and technical requirements, it is allowed to apply for filing.
Application process for the qualification of the original registrant
-
Products that have been registered:

Specific instructions for the application process:
1) The registration applicant submits a “registration transfer to filing” change application in the health food registration system;
2) Obtain the “Notice of Evaluation Opinion”.
-
If the product registration applications including new product registration, renewal registration, change registration and transfer technology registration are accepted before the release of the Health Food Raw Materials Directory, the applicants can choose the following two ways to obtain the original registrant's qualification:
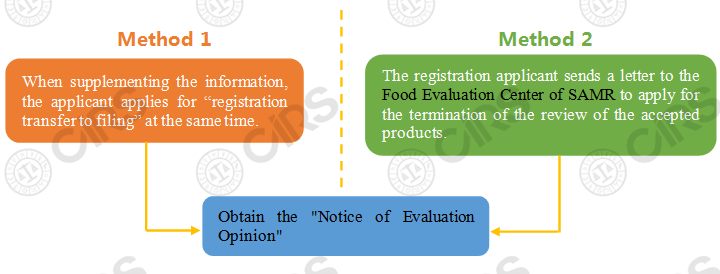
Note: Regarding the Method 1, after the Food Evaluation Center of SAMR issues a request for supplementing information to the applicant, the applicant can apply for “registration transfer to filing” when supplementing information. For products that meet the filing requirements, the Food Evaluation Center of SAMR generally no longer requires supplementary information, but directly issues a “Notice of Disapproval”. When the applicant received the notice, it means that the applicant obtains the original registrant’s qualifications. When the method is adopted, it is necessary to wait for the notice of opinion issued by the Food Evaluation Center of SAMR, and the applicant's situation is relatively passive.
Dossier requirements for registered products
Domestic health foods
1) Domestic health food change application form, letter of commitment for authenticity of the materials;
2) Copies of legally registered certificates of the applicant;
3) Copy of health food registration certificate and its attachments;
4) The specific matters, reasons and basis of the change;
5) Application form for registration transfer to filing.
Imported health foods
1) Imported health food change application form, letter of commitment for authenticity of the materials;
2) Copies of legally registered certificates of the applicant;
3) Copy of health food registration certificate and its attachments;
4) Qualification certifying documents issued by the government authorities or legal service agencies in the producing country (region) of origin proving that the overseas applicant is the owner of the health food marketed;
5) Certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the product has been marketed more than a year, or safety report of overseas sales and consumer’s feedback;
6) If the product also needs to apply for approval in producing country (region), the proof document issued by the government authorities is needed.
7) Health food-associated standards issued by the product producing country (region) of origin or international organizations;
8) Packaging, labels, package inserts for products marketed in the producing country (region) of origin;
9) For registration affairs run by oversea manufacturer’s Permanent Representative in China, a copy of the “registration certificate of oversea enterprise’s permanent Chinese representative offices” shall be provided; for registration affairs run by domestic agencies entrusted by oversea manufacturers, the applicant shall provide the original notarized certificate of entrustment and copies of business license of the agencies.
10) The specific matters, reasons and basis of the change;
11) Application form for registration transfer to filing.
2. Apply for health foods filing
-
Domestic health foods: apply for the filing system login account (except for those with a login account) or product filing as an original registrant.
-
Imported health foods: product filing application policy has not been clear.
According to the Q&A about how to apply for filing by “registration transfer to filing” issued by Food Evaluation Center of SAMR on July 5, 2017, the filing application for imported products is the same as it for domestic products. However, CIRS found that there is no application entry for health foods that use one of the five substances as single active ingredient in the imported health food filing system. According to the communication with the experts of the Food Evaluation Center of SAMR, the competent authorities are still discussing that, in what ways overseas applicants can apply for product filing by “registration transfer to filing”.
CIRS will continue to pay attention to the latest policy changes in the filing of imported health foods with the five raw materials including Coenzyme Q10, Melatonin, Fish oil, Broken Ganoderma lucidum spore powder and Spirulina. For imported registered products that use one of the five substances as single active ingredient, a series of certification documents shall be provided for the confirmation of original registrant, and the period for the applicant to prepare these certification documents is generally long. Therefore, CIRS recommends that overseas companies prepare in advance to apply for the qualifications of the original registrant, to ensure that enterprises can get the filing certificate quickly after the policy is determined.
If you have any needs or questions, please contact us at service@jianzaoshiwang.cn.

