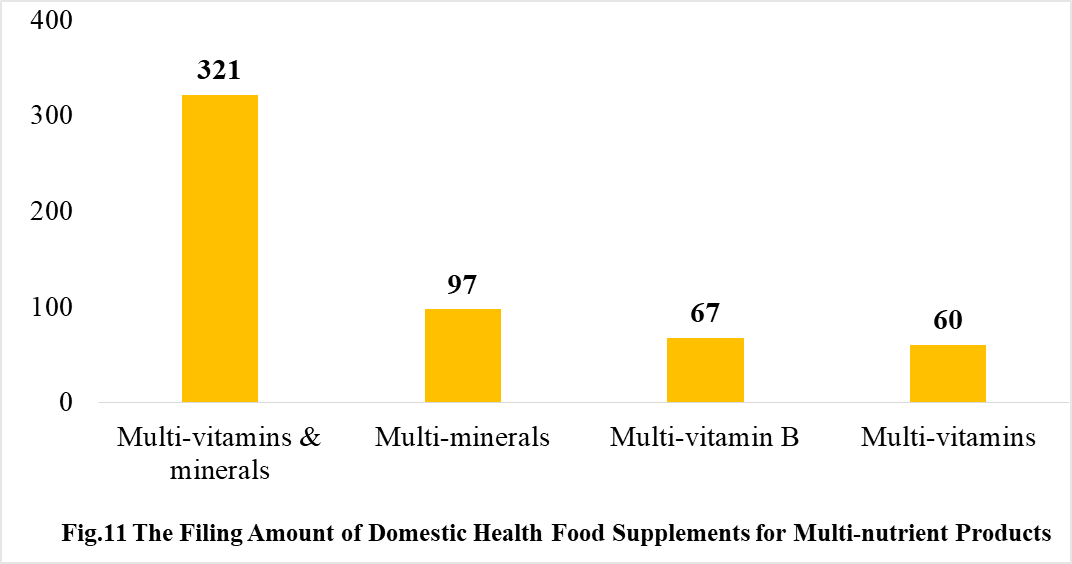Updated on 14 Feb. 2022
In order to help enterprises better understand the filing status of health food (dietary supplement) in China, CIRS counted the data of filed products published in 2020 and made an analysis for your reference.
1. The Filing Status of Health Food in China
According to the information released by the Center for Food Evaluation of SAMR and the Local Administration for Market Regulation, a total of 1840 health food obtained the filing certificates, of which 1777 are domestic health foods and 63 are imported health foods by December 31, 2020.
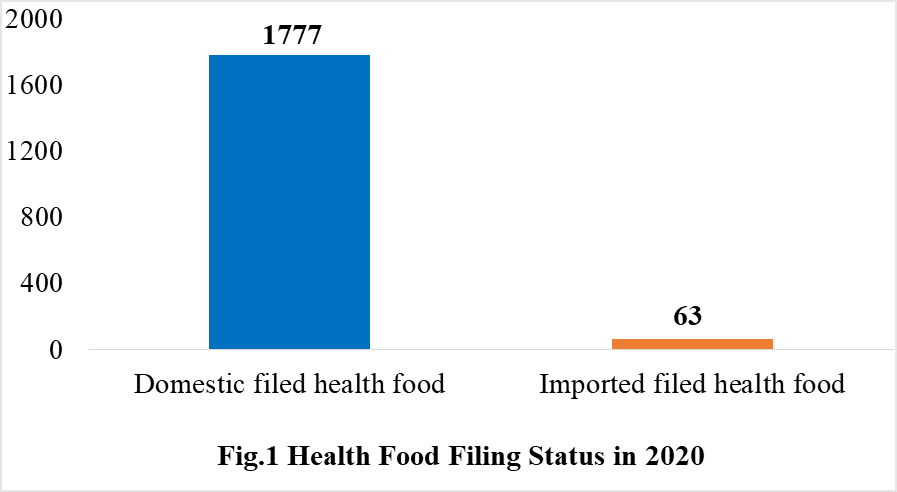
2. The Filing Status of Health Food in Different Regions
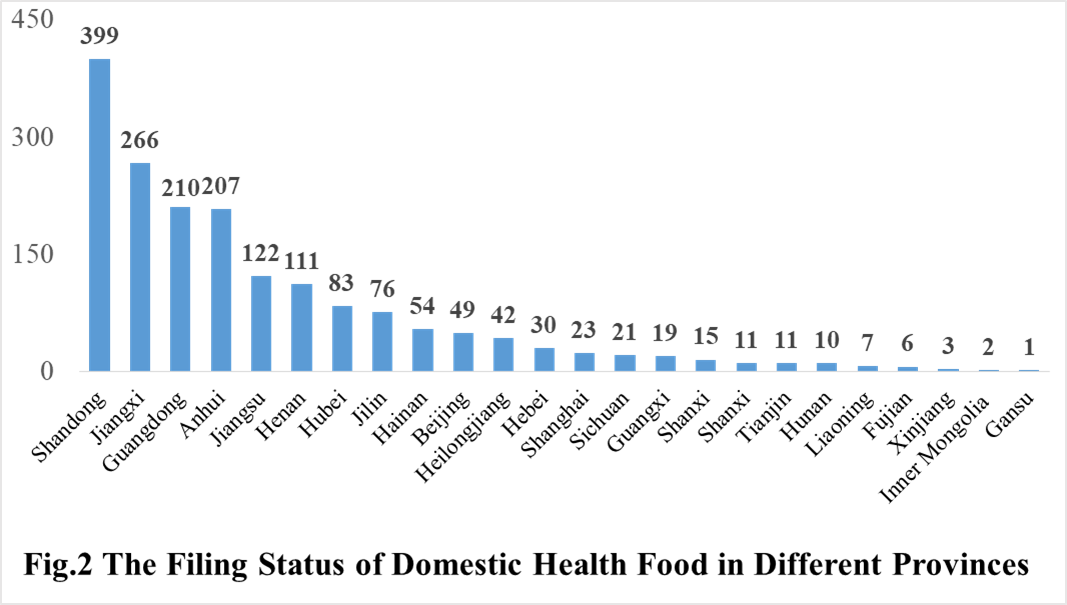
The number of approved domestic filing health food are varied in different provinces in China. Shandong and Jiangxi rank the first and second place respectively with the number of 399 and 266, followed by Guangdong province (210). And the applicants who come from the United States, Australia, Canada, Japan, Germany, Denmark and Taiwan-China have obtained filing certificates, and 39 of total approved imported nutrition supplements are from American companies.
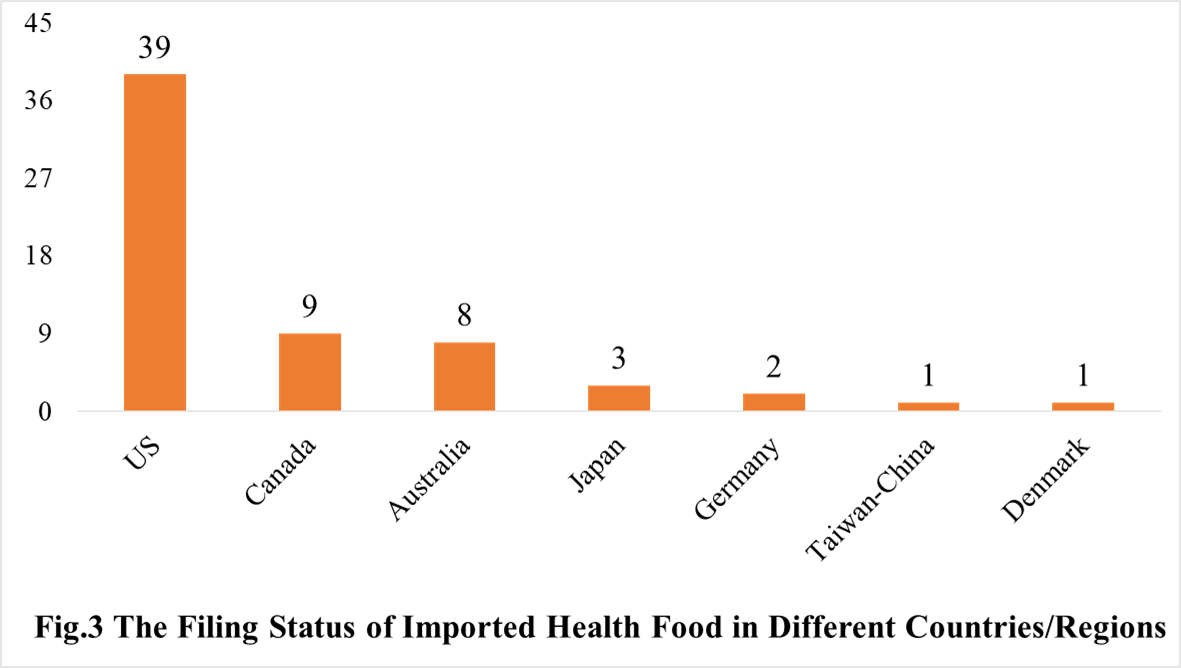
3. The Filing Status of Health Food in Different Enterprises
3.1 Domestic Filing Products
In 2020, 298 domestic health food manufacturers have obtained health food filing certificates. Among them, Weihai Baihe Biology Technology Co., Ltd. has got the largest number of filing certificates (156), followed by Wuhan Maixinli Pharmaceutical Co., Ltd. and Jiangxi Haoyu Biotechnology Co., Ltd. with the number of 45 and 42, respectively.
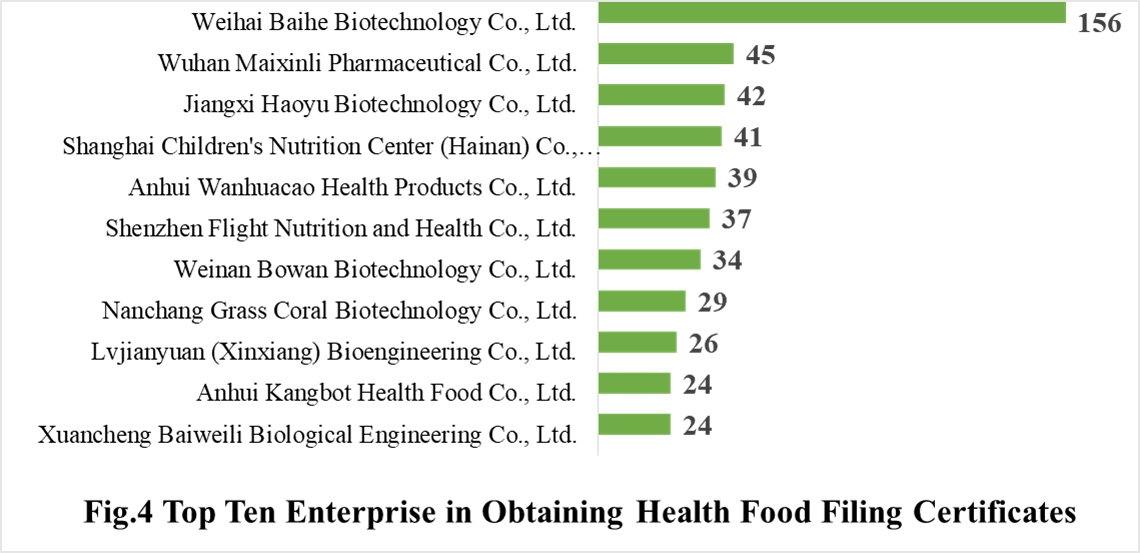
3.2 Imported Filing Products
In 2020, 17 oversea companies have got health food filing certificates, USA BORNATOP INVESTMENT & MANAGEMENT CO., LTD has got the largest number of filing certificates (11). Jamieson Laboratories Ltd. ranks the second place with the number of 9 products, followed by DOCTOR'S CLINICAL, INC. with the number of 7 products.
4. The Filing Status of Health Food in Different Dosage Forms
There are currently 5 dosage forms for nutrition supplement which are available for filing certificate in China, namely, tablet, hard capsule, soft capsule, oral liquid (including drops) and granule.
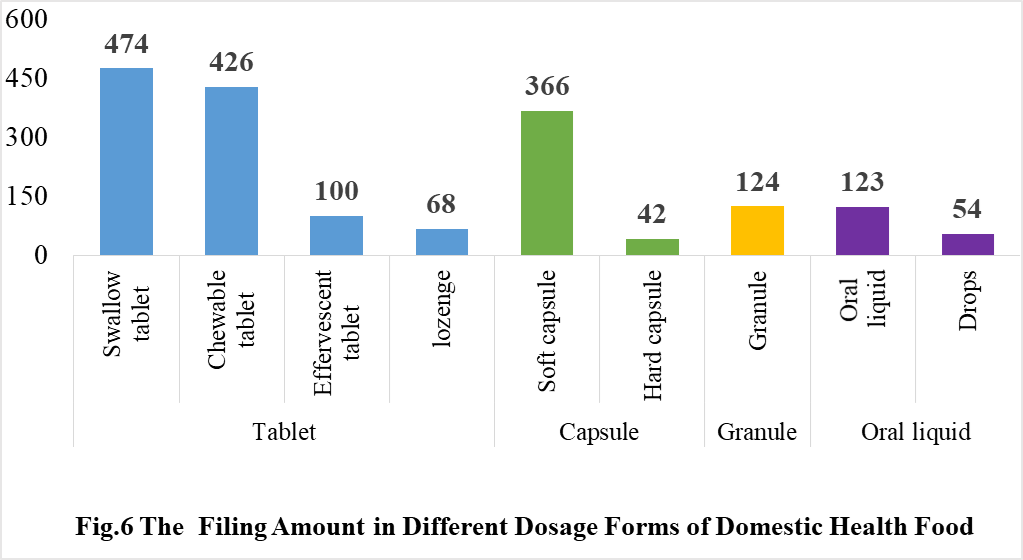
4.1 Filing status of domestic products
In the 2020, the most popular dosage form for domestic health food filing is tablet with the number of 1068, accounting for 60.10% of the total.
The capsule include soft capsule and hard capsule, and the number of approved products is 408. The quantity of soft capsule products is higher than the number of hard capsule products, which are 366 and 42 products respectively.
In addition, the quantity of oral liquid (including drops) and granule products are 177 and 124 respectively. Among them, only 54 products are drops.
4.2 Filing status of imported products
In 2020, the dosage form of approved imported health foods is mainly tablet with the number of 37. The number of capsule and oral liquid (including drops) products is 11 and 15 respectively. There is no granular products in 2020.
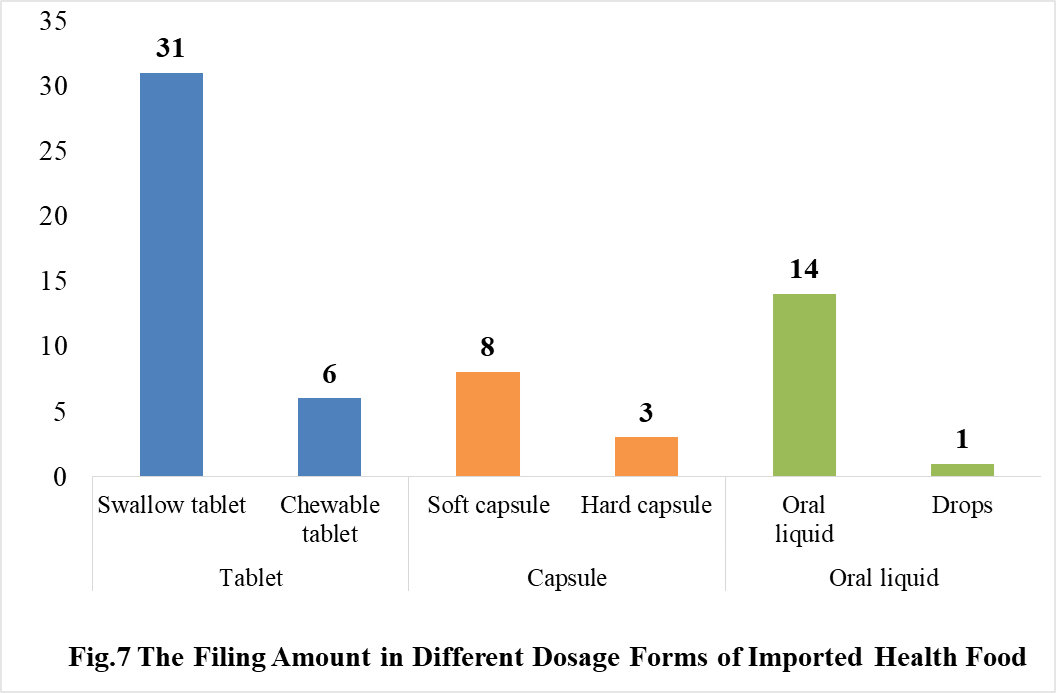
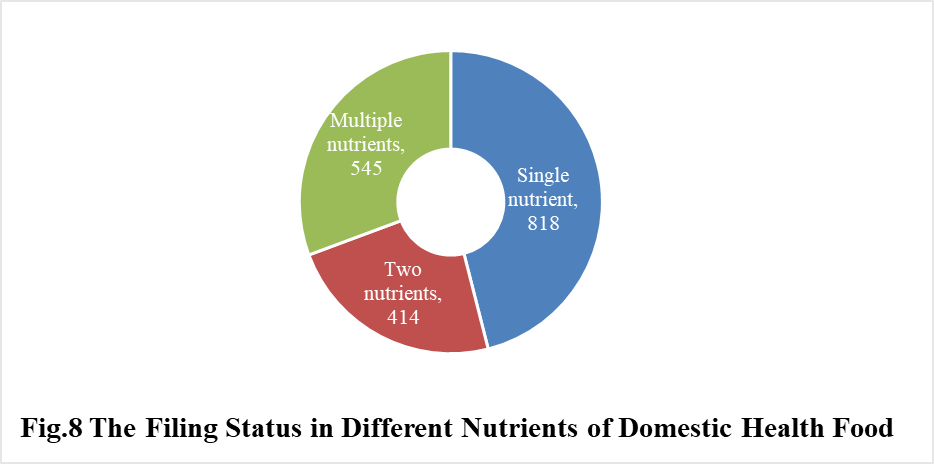
5. The Number of Approved Filed Health Food with Different Nutrients
5.1 Domestic Filing Products
In 2020, the number of domestic products supplementing single nutrient is the most, which is 818, followed by the products supplementing multiple nutrients and two nutrients, the number is 545 and 414 respectively.
Among the domestically filed products in 2020, the most popular nutrition supplements are multi-vitamins and minerals supplements, Vitamin C supplements, Calcium and Vitamin D supplements, which is 321, 309 and 138 respectively.
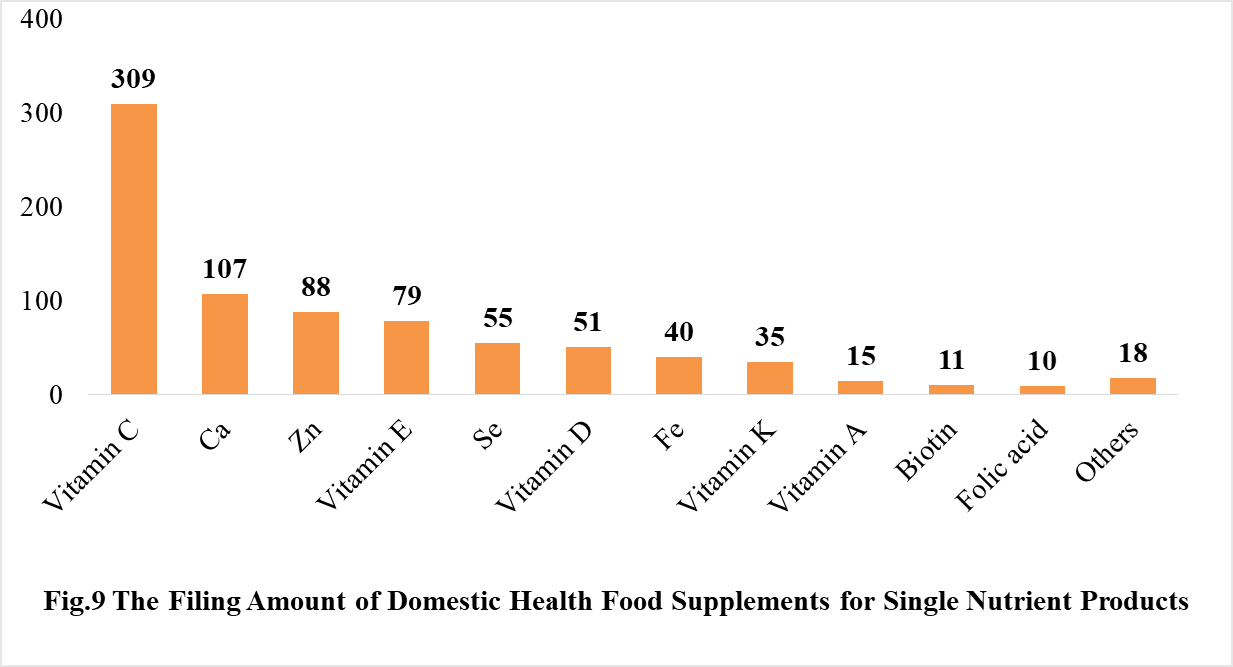
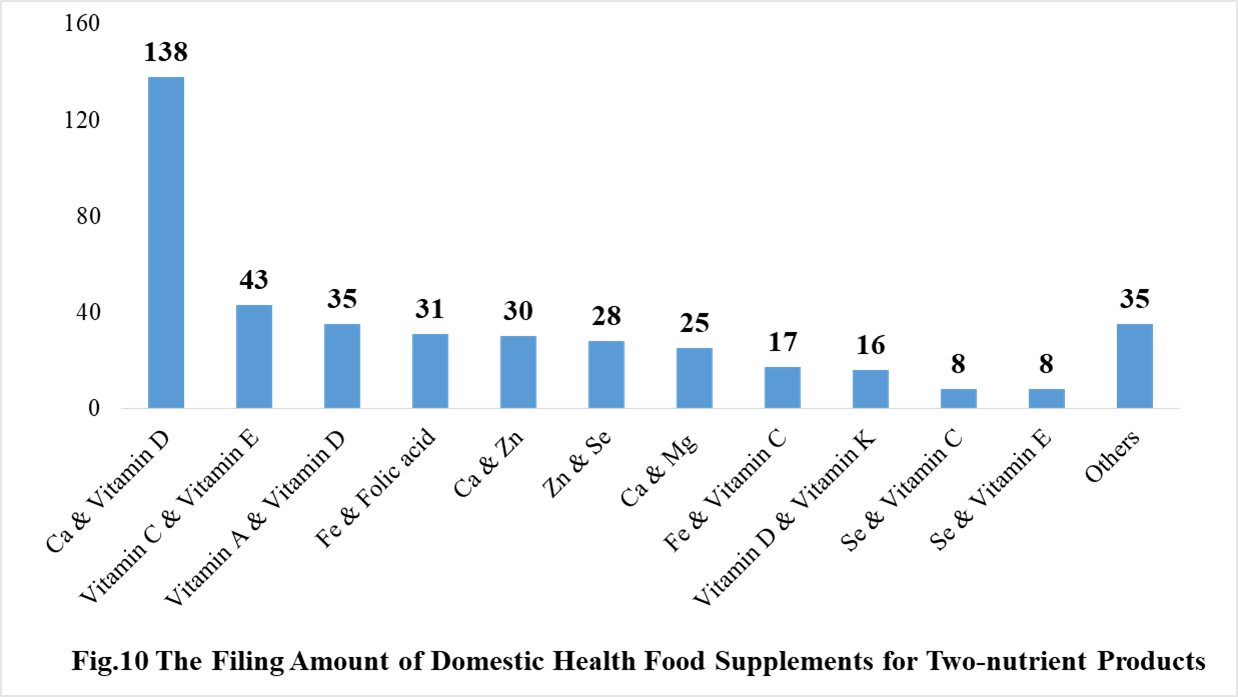
5.2 Imported Filing Products
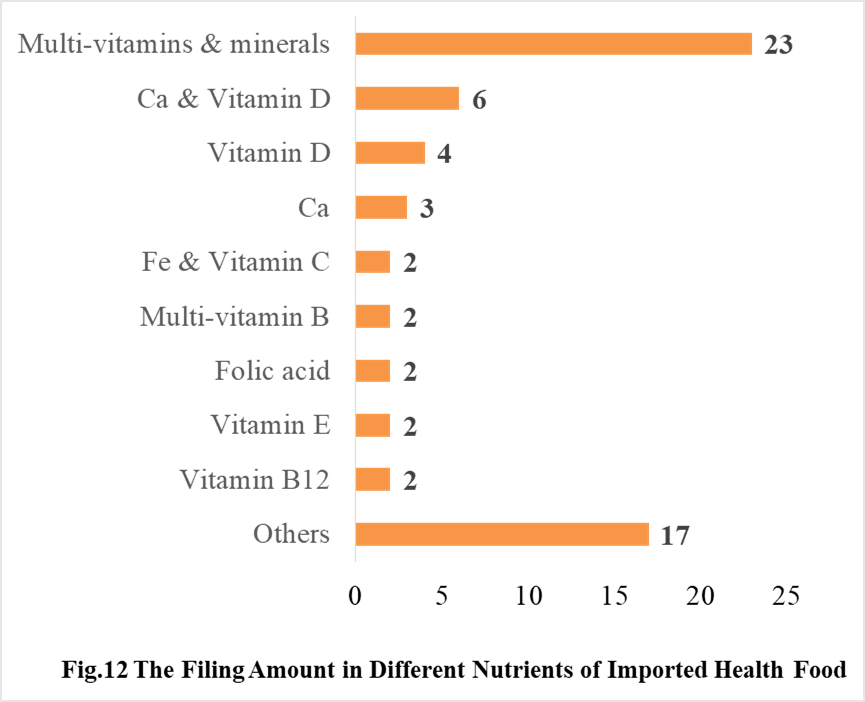
In 2020, the number of imported products supplementing Multi-vitamins & minerals is the most, which is 23, followed by the products supplementing Ca & Vitamin D and Vitamin D, which is 6 and 4 respectively.
CIRS Comments
The implementation of the health food filing has simplified the procedures of administrative approval and reduced the reporting period and costs, so it is favored by health food companies. From 2017 to 2020, more than five thousand nutrition supplements have obtained filing certificates, while only 135 imported products have got filing certificates, which could result from the lack of fully understanding of Chinese laws and regulations related to health food, and the fact that the relevant supporting documents provided by the applicants sometimes cannot meet the requirements of the Chinese laws and regulations. The article "Registration and development trend of imported health food in China" written by the specialist of the Center for Food Evaluation of SAMR stated that the qualification of overseas enterprises, production and sales certificates of products etc. are the important certificates to guarantee that the products have been legally produced and sold overseas. In the future, these relevant supporting documents will still be the focus of the review on the materials of imported filing products.
For health food enterprises, 2020 is a year full of good news. Health Food Raw Materials Directory of Nutrition Supplement (2020 version), List of Health Functions Available for Nutrition Supplement (2020 version) and Health Food Raw Material Directory of Coenzyme Q10, Melatonin, Fish oil, Broken Ganoderma Lucidum Spore Powder and Spirulina will come on effect on 2021.03.01. After the implementation of the above regulations, enterprises will have more choices on raw materials and function claims for filing products, and the types of health food filing products in the market will also increase.
Note:
I. The data in this article is from the Special Food Information Query Platform, Center for Food Evaluation of SAMR, and Local Administration for Market Regulation.
II. There may be some omissions in the data of domestic filed health food due to the replacement of new and old websites of government departments after the reform of state institutions, thus the data in this article is for reference only, and please refer to the information published by the government.
III. The Special Food Information Query Platform and Center for Food Evaluation of SAMR lag behind in information release. According to CIRS’s knowledge, the information of some products that have obtained health food filing certificates have not been published on the official website of the government departments. Therefore, the actual record amount of health food exceeds the data listed in this article.
If you have any needs or questions, please contact us at service@jianzaoshiwang.cn.