24th
April 2015,
the strictest food safety law
was published by the National People’s Congress Standing Committee, it stipulates that the formula of infant formula milk powder should be registered under China Food and Drug Administration (CFDA). Then, on 2nd
September 2015, CFDA released a Notice for public comments on the
Administrative measures for the registration of formula of infant formula milk powder (exposure draft), and opinions and advice should be given before 1st
October 2015. According to this draft, the management of the product formula of infant formula milk powder will upgrade from record to registration, and also one formula could only be used to produce one product by the same company.
Application Scope
This regulation is applicable for the registration of product formula of infant formula milk powder which is produced in China.
What is the product formula of infant formula milk powder?
The product formula of infant formula milk powder means all the raw materials and their dosages, and the product’s nutrition content.
Who should apply for the registration of product formula of infant formula milk powder?
The applicant should be the manufacturer of the infant formula milk powder in China.
Who should be responsible for the management of the registration of product formula of infant formula milk powder?
China Food and Drug Administration (CFDA) should be responsible for the management of the registration of product formula of infant formula milk powder.
What are the required materials?
Application Scope
This regulation is applicable for the registration of product formula of infant formula milk powder which is produced in China.
What is the product formula of infant formula milk powder?
The product formula of infant formula milk powder means all the raw materials and their dosages, and the product’s nutrition content.
Who should apply for the registration of product formula of infant formula milk powder?
The applicant should be the manufacturer of the infant formula milk powder in China.
Who should be responsible for the management of the registration of product formula of infant formula milk powder?
China Food and Drug Administration (CFDA) should be responsible for the management of the registration of product formula of infant formula milk powder.
What are the required materials?
- Application
- Formula research report and production process specification
- Product testing report
- Evidentiary materials of the production, research and testing capabilities
- Sample manuscript of the label and specification
- Other materials to prove the scientificity and safety of the formula
How many formulas could be registered by one company?
Currently, there are two ways to limit the quantity of registered formula of one company.
1. There should have a significant difference between each registered product formula which is produced by the same company and for the same age. The optional ingredients stipulated by national food safety standards should differ by at least 6 ingredients and it should be confirmed by scientific basis.
2. There should have a significant difference between each registered product formula which is produced by the same company and for the same age. It should be confirmed by scientific basis, and there are at most 5 series 15 kinds of formulas for one company.
Contents of the certificate
- Company name
- Company address
- Product name
- Product formula
- Product label and specification
- Approval number
- Approval date
- Effective
- Validity period
Registration procedure of the formula of infant formula milk powder
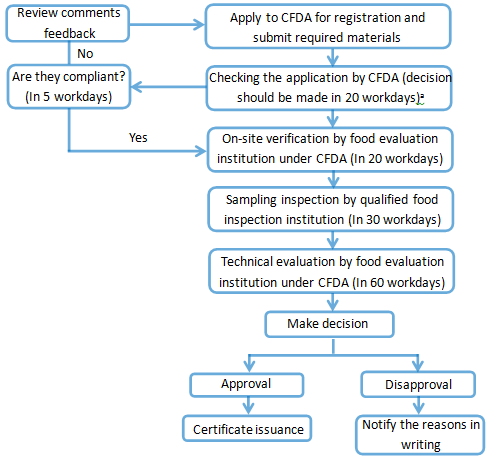
Ps. ‘a’: The time of on-site verification, sampling inspection and technical evaluation is not included in the 20 workdays.
Requirements of label and specification
Compared with common prepackaged food, requirements of the label of infant formula milk powder is stricter. It should comply with the specific requirements stipulated by this draft, besides the basic requirements listed in GB 7718-2011 General rule forthe labeling of prepackaged food and GB 28050-2011 General rule for the nutrition labeling of prepackaged food . Please kindly find below table for detailed information.
Table 1 Specific requirement for the labeling of infant formula milk powder
| No. | Requirements |
|---|---|
| 1 | The label and specification should be the same as what listed in registration certificate. |
| 2 |
The product name could be indicated as ‘嬰幼兒配方奶粉(infant formula milk powder)’, and it should be labeled next to the brand with the maximum font seize except the brand. If the product is made from ewe’s milk (powder), the product name could be indicated as ‘嬰幼兒配方羊奶粉 (infant formula milk powder from ewe’s milk)’. Besides, the content of ewe’s milk (powder) per 100g product and the source of whey protein powder should be labeled in the ingredients list. |
| 3 | Infant formula milk powder includes infant formula (0-6 months, stage 1), older infant formula (6-12 months, stage 2) and young children infant formula (12-36 months, stage 3). Applicable months of the product should be labeled, and ‘1, 2, 3’, ‘1段,2段,3段 (stage 1, stage 2, stage 3)’ could be indicated at the same time. |
| 4 | The specific name of edible vegetable oil should be indicated within the following parenthesis, and each vegetable oil should be listed in descending order of their contents. |
| 5 |
The name, address and contact information of the actual manufacturer should be labeled. Meanwhile, the name and address of the group company could also be labeled. Production date, expiration date and etc. should be highlighted. |
| 6 | The formula approval number should be labeled. |
| 7 |
If any ingredient is emphasized as imported, its country of origin should also be labeled. Fuzzy words which may mislead customer are forbidden, such as ‘進口奶源 (import milk)’, ‘源自國外牧場 (from foreign ranch)’ etc. |
| 8 | The content, style and color of the label and specification of the products which are produced with the same formula by the same company should keep unified. |
| 9 |
Following contents are forbidden: a. Express and imply with the function of disease prevention or treatment. b. Express and imply with the function of improving intelligence, strengthening resistance or immunity, protecting intestine and health care. c. Recommended or supervised and etc. by social organization, such as industries association, customer organization, inspection institution and etc. d. Words like ‘GMO-free’. e. Words like ‘zero- added’. d. Other contents forbade by laws, regulations and national food safety standards. |
| 10 |
Mandatory content of the label should be in a contrast color from the background, and the brightness contrast should be more than 70%. Warning or notice should be labeled with different font and color, and the min-height is 3.7mm. |
According to incomplete statistics, there are more than 2000 brands regarding infant formula milk powder and more than 2000 recorded formula in China now, but there is very little difference between the formulas of different products. And experts predict that the quantity of the brand will reduce to about 400 after the draft come into force. The new draft will have a significant impact on the dairy industry. Meanwhile, although there is no limit for the imported infant formula milk powder in this draft, according to sources, detailed administrative measures regarding formula registration of imported infant formula milk powder will be published by AQSIQ in the near future, Relevant enterprises should be prepared and CIRS will keep focusing on the follow-up progress.
Reference
http://www.sda.gov.cn/WS01/CL0782/128400.html
Contact us
Ms. Alice Yang, Food & Health Products, CIRS China
11F Dongguan Building, 288 Qiuyi Road, Binjiang District, Hangzhou, China, 310020
Tel: +86 571 8720 6555 | Fax: +86 571 8720 6533
Email:Alice.Yang@jianzaoshiwang.cn






