The European Commission has recently published regulation (EU) 2023/464, which amends Annex Regulation (EC) No 440/2008, a set of test methods under the Registration, Authorization and Restriction of Chemicals (REACH) regulation.
In this amendment, a number of new OECD in vitro test methods are introduced while some old test methods are removed such as Two-Generation Reproduction Toxicity Study (OECD 416) and Unscheduled DNA Synthesis (UDS) Test with Mammalian Liver Cells in vivo (OECD 486), which promotes the application of in vitro test methods in the EU.
Based on this amendment, in the following article, we have summarized the significant changes in test methods based on the testing requirements of the REACH regulation.
1. Serious eye damage/eye irritation
OECD Test Guideline 467, Defined Approaches for Serious Eye Damage and Eye Irritation, is introduced. With OECD 467, categories of test chemicals can be made based on in vitro tests without conducting further in vivo tests in most cases. In the following flowchart, one of the methods from OECD 467 is given. When conducting tests, the physiochemical properties of test chemicals, such as water solubility, vapor pressure, Kow, the physical state of test chemicals, and surface tension, should be taken into account. With the combination of OECD 492 and OECD 437, category prediction of test chemicals can be made as Not classified, Category 1, or Category 2. One can also combine OECD 491 with OECD 437 to make category prediction of test chemicals as Not Classified, Category 1, or Category 2.
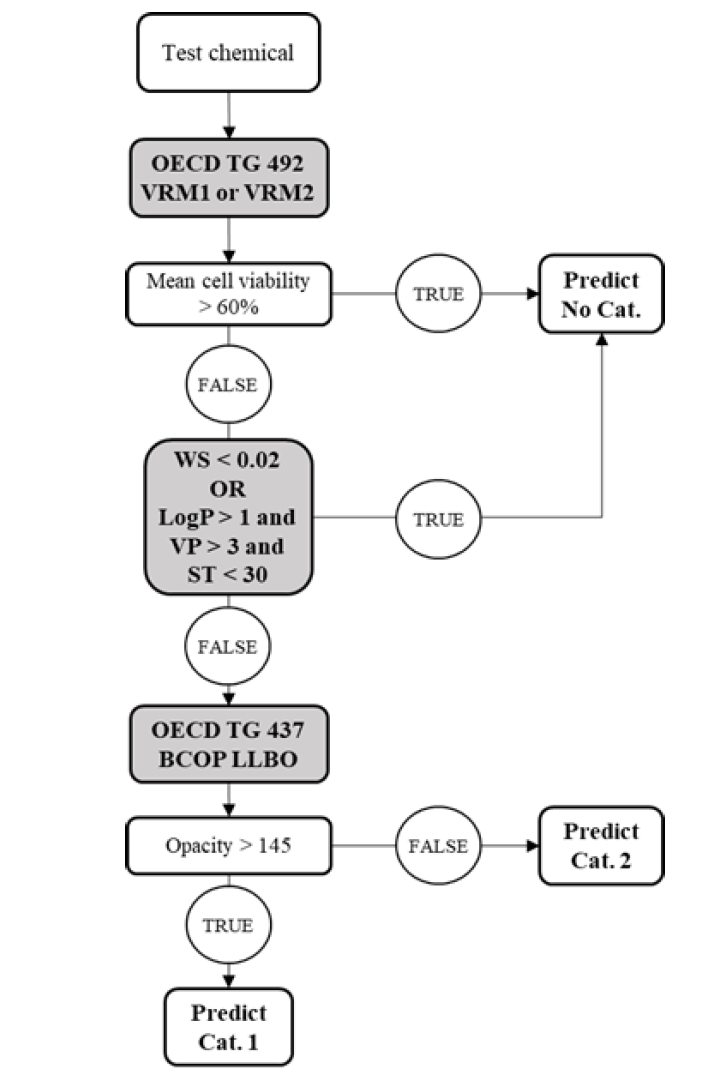
2. Skin sensitization
OECD Test Guideline 497, Defined Approaches on Skin Sensitization, is introduced. With OECD 497, skin sensitization can be predicted by using two in vitro tests (OECD 442C and OECD 442E) plus QSAR results. If the results of in vitro tests are reasonable, a category of test chemicals can be made based on the total score of in vitro tests plus the QSAR result, as shown in the following flowchart. If both in vitro tests or the QSAR results are not reasonable, there is still a possibility of predicting the category of test chemicals. These test methods can be conducted to significantly reduce the Local Lymph Node Assay (LLNA) testing.
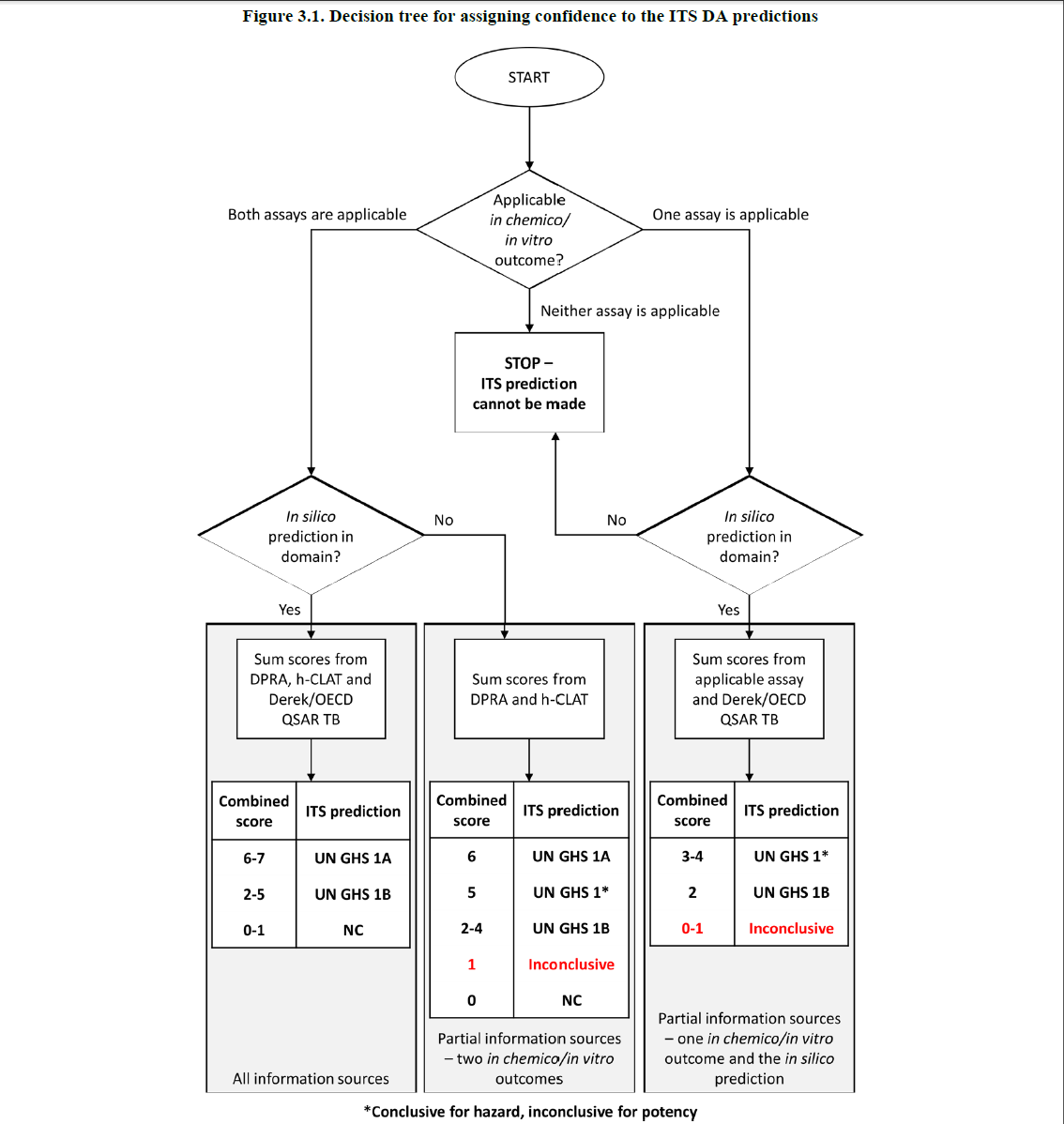
3. Mutagenicity
OECD Test Guideline-488 Transgenic Rodent Somatic and Germ Cell Gene Mutation Assays and OECD 470-Mammalian Erythrocyte Pig-a Gene mutation Assay can be adopted to give more options for in vivo gene mutation experiments. Moreover, Unscheduled DNA Synthesis (UDS) is removed. Currently, in the dossier evaluation of the REACH Regulation, either of Comet or Transgenic test is required for in vivo gene mutation assays.
4. Reproductive/developmental toxicity
OECD 443: Extended One-Generation Reproduction Toxicity Study (OECD 443) is introduced to take the place of OECD 416: Two-generation reproduction toxicity study, which was earlier mandated in REACH registration. However, the high cost of the test may cause significant financial pressure for registrants involving high tonnage registration, as some laboratories in Europe have quoted test prices as more than a million euros.
5. Endocrine-disrupting properties
A series of new in vitro test methods for endocrine disrupting properties are newly added, such as OECD 455: Performance-Based Test Guideline for Stably Transfected Transactivation In Vitro Assays to Detect Estrogen Receptor Agonists and Antagonistsals; OECD 456: H295R Steroidogenesis Assay; OECD Test Guideline 458: Stably Transfected Human Androgen Receptor Transcriptional Activation Assay for Detection of Androgenic Agonist and Antagonist Activity of Chemicals; OECD 493: Performance-Based Test Guideline for Human Recombinant Estrogen Receptor (hrER) In Vitro Assays to Detect Chemicals with ER Binding Affinity, and so on. In vitro testing methods such as OECD 440: Uterotrophic Bioassay in Rodents A short term screening test for oestrogenic properties and OECD 441: Hershberger Bioassay in Rats, A Short Term Screening Assay for (Anti) Androgenic Properties are also introduced. Since endocrine-disrupting classification is introduced in the amended CLP regulation, it is anticipated that tests for endocrine-disrupting characteristics (EDCs) may be regarded as an important factor in assessing the property of test chemicals.
Other test methods for toxicology and ecotoxicology will not be elaborated here as they are not commonly used and therefore have a tiny impact on the selection of tests.
CIRS Comments
As we can see from this amendment, the EU is advancing the in vitro test methods especially the world-leading test methods for skin corrosion/irritation, serious eye damage/irritation and skin sensitization. These in vitro test methods are expected to expand to other countries and further promote the application of alternative test methods in the worldwide range. In the meantime, the EU has amended the CLP regulation and introduced EDCs. Both in vitro and in vivo test methods for EDCs may be combined together to identify the endocrine-disrupting properties of test chemicals, which shall attract the attention of enterprises.
The newly added in vivo genotoxicity tests including OECD 488 and OECD 470 provide more available test options. As these tests are novel and expensive, it is arduous to select appropriate laboratories since these tests have stricter requirements. Since OECD 416 has been replaced by an extended one-generation reproductive toxicity study, it will not have an influence on REACH registration and may cause greater financial pressure on relevant registrants as OECD 443 test is costly.
To sum up, when carrying out REACH registration, enterprises should refer to the latest guidelines and select appropriate test methods to avoid any potential noncompliance issues.
If you need any assistance or have any questions, please get in touch with us via service@jianzaoshiwang.cn.

