On November 12, 2024, the Scientific Committee on Consumer Safety (SCCS) of the European Union issued a preliminary opinion on Ethylhexyl Methoxycinnamate (CAS No. 5466-77-3/83834-59-7). The deadline for comments on SCCS/1671/24 is set for January 17, 2025.

The SCCS concludes the following:
Having considered the data provided and the concerns relating to potential endocrine-disrupting properties of EHMC, the SCCS cannot conclude on the safety of EHMC, because the information provided is insufficient to exclude genotoxicity.
In addition, the available evidence also shows that EHMC is an endocrine-active substance due to a clear demonstration of estrogenic activity and weak anti-androgenic activity both in vitro and in vivo.
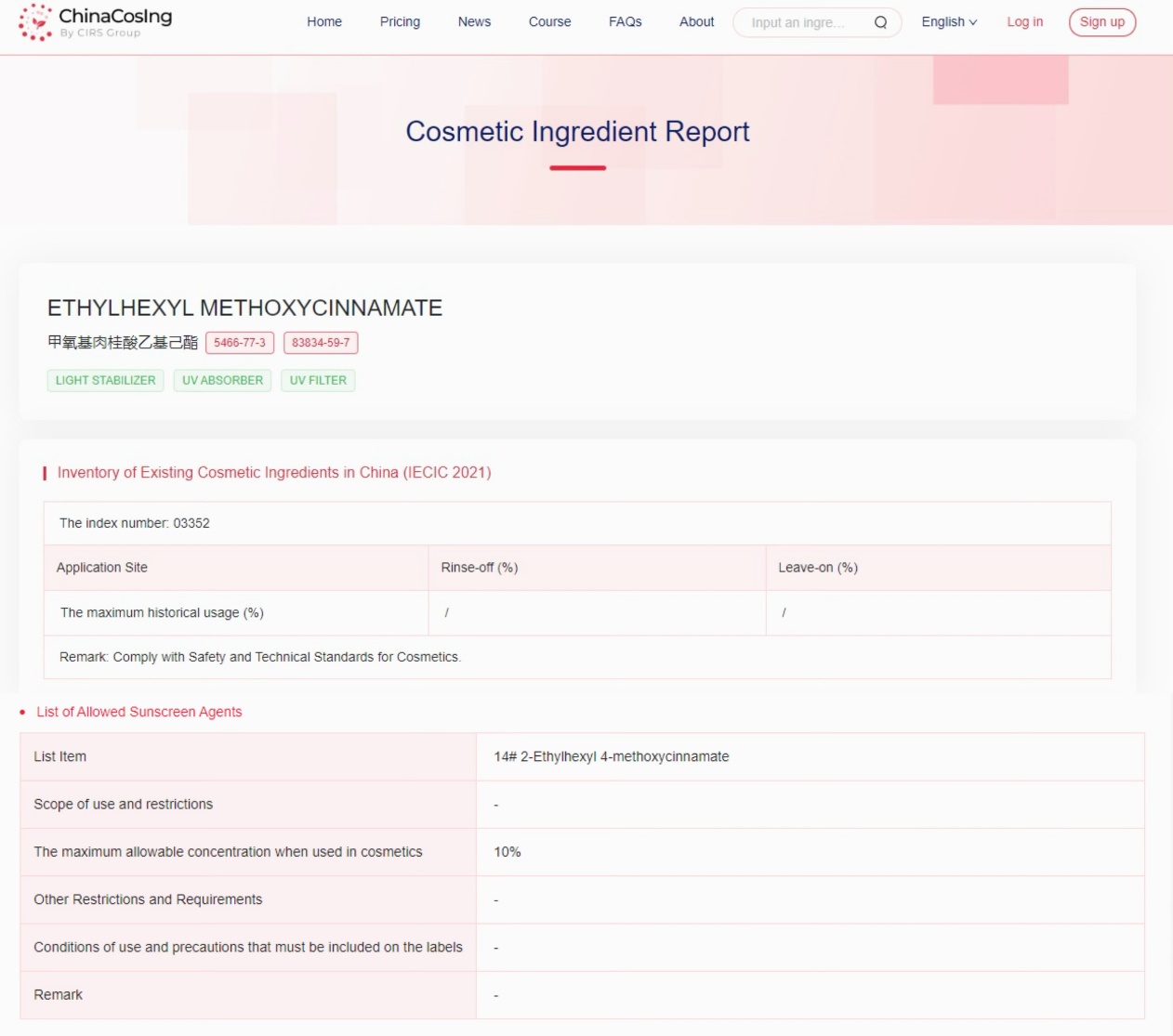
The Chinese Cosmetic Ingredient Regulatory Database (ChinaCosIng), independently developed by CIRS Group, indicates that Ethylhexyl Methoxycinnamate, used as a light stabilizer, UV absorber, and UV filter, has been included in the "Inventory of Existing Cosmetic Ingredients in China" (IECIC) (2021 edition) and the "List of Allowed Sunscreen Agents", with the maximum allowable concentration when used in cosmetics being 10%.
If you need any assistance or have any questions, please get in touch with us via service@jianzaoshiwang.cn.
Further Information

