In the first half of 2022, the National Health Commission of the People's Republic of China (NHC) issued two announcements (No.1 of 2022; No.2 of 2022) in the form of ‘Three New Food’ with a total of 68 products approved, including new food raw materials (novel food), new food additives, and food-related products (new food contact substances). Among them, 5 new food raw materials (novel food) were approved.
Besides, the websites of NHC and China National Center for Food Safety Risk Assessment (CFSA) show that a total of 8 new food raw materials applications were accepted, 2 of public comments on ingredients were issued, and 1 ingredient review were terminated in the first half of 2022.
CIRS will take you to the detailed information about the acceptance and approval of new food raw materials in China in the first half of 2022 as following:
1. List of new food raw materials application acceptance in the first half of 2022 (8 types)
In the first half of 2022, NHC accepted the 8 applications of new food raw materials, including 7 domestic products and 1 imported product.
S/N | Acceptance Date | Acceptance Code | Acceptance Name |
1 | 17/01/2022 | 衛食新申字(2022)第0001號 | Penthorum chinense Pursh. |
2 | 17/03/2022 | 衛食新申字(2022)第0002號 | (3R,3’S)-dihydroxy-β-carotene |
3 | 29/03/2022 | 衛食新進申字(2022)第0001號 | Maqui berry Extract |
4 | 24/04/2022 | 衛食新申字(2022)第0003號 | Trichosanthis Semen |
5 | 07/05/2022 | 衛食新申字(2022)第0004號 | Peach gum |
6 | 23/05/2022 | 衛食新申字(2022)第0005號 | The pine needles of Pinus massoniana |
7 | 06/06/2022 | 衛食新申字(2022)第0006號 | Shiny-leaved yellowhorn leaf |
8 | 09/06/2022 | 衛食新申字(2022)第0007號 | Brown sugar fructooligosaccharides |
2. List of drafted approved new food raw materials in the first half of 2022 (2 types)
In the first half of 2022, Arabinoxylan and Leuconostoc pseudomesenteroides passed the technical review of CFSA and were issued for public comments.
2.1 Arabinoxylan (Draft announcement)
Name | Arabinoxylan |
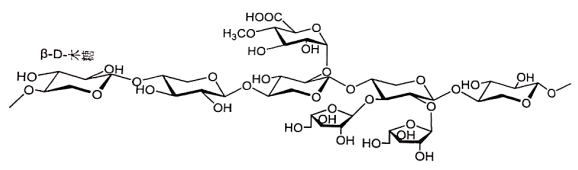
Basic information | Structural formulas (fragment): CAS Reg. No. 9040-27-1 |
Brief introduction of production process | It is made from gramineous plants such as bagasse, wheat bran, corn husk, etc., through pretreatment, edible alkali extraction, dealkalization concentration, neutralization precipitation, washing purification, drying and other processes. |
Recommended daily amount | ≤15 g/ day (Based on the arabinoxylan content of 85 g/100 g, the content exceeding the actual content shall be converted) |
Other information | 1. Infants, young children, pregnant women and lactating women are not suitable for consumption. The labels and instructions shall indicate the unsuitable population. 2. See appendix for quality specifications and food safety indicators. |
Issued date | 14/03/2022, CFSA Opened Public Comment for Arabinoxylan |
2.2 Leuconostoc pseudomesenteroides (Draft announcement)
Name | Leuconostoc pseudomesenteroides |
Other information |
|
Issued date | 29/06/2022, CFSA Opened Public Comment for Leuconostoc pseudomesenteroides |
Acceptance date | 19/04/2021, 衛食新進申字(2021)第0002號 |
3. List of approved new food raw materials in the first half of 2022 (5 types)
At the end of June, there were 5 new food raw materials approved, the detailed approval information are as follows:
3.1 Kanzan flower
Name | Kanzan flower |
Basic information | Species: Cerasus serrulate ‘Sekiyama’ Edible parts: flower The flowering period of collection is from more than 3/4 of the buds to full bloom. |
Other information | 1. Infants, young children, pregnant women and lactating women are not suitable for consumption. The labels and instructions shall indicate the unsuitable population. 2. Food safety indexes are implemented in accordance with the relevant national regulations of other vegetables products. |
Acceptance date | 25/01/2021, 衛食新申字(2021)第0001號 |
Issued date | 17/08/2021, CFSA Opened Public Comment for Kanzan flower and Pyrroloquinoline quinone disodium (PQQ) salt |
Approved date | 01/03/2022, officially approved by Announcement No.1 of 2022 |
3.2 Pyrroloquinoline quinone disodium (PQQ) salt
Name | Pyrroloquinoline quinone disodium(PQQ)salt | |
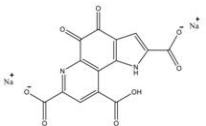
Basic information | CAS: 122628-50-6 Molecular formula:C14H4N2Na2O8 Molecular weight:374.17 Structure: | |
Production process | Using 6-amino-5-methoxyindole-1H-2-carboxylic acid ethyl ester and dimethyl 2-oxoglutaconate as raw materials, after coupling reaction, formation of quinoline ring, oxidation reaction and hydrolysis reaction of ester and refining. | |
Recommended intake | ≤20 mg/d | |
Quality requirement | Property: Reddish brown powder | |
Pyrroloquinoline quinone disodium(PQQ)salt content(on dry basis)≥98.0 g/100 g | ||
Moisture ≤12.0 g/100 g | ||
Other information | 1. Application scope and maximum usage: beverages (40 mg/kg, solid beverages are converted according to the mass of liquid after preparation). 2. The substance shall not be consumed by infants, young children, pregnant women and lactating women. Inapplicable population should be marked on labels and instructions. 3. Pyrroloquinoline quinone disodium(PQQ)salt and the other two impurities’ test method check the link. 4. Food safety indicators must meet the following requirements: | |
Pb/(mg/kg) | ≤0.5 | |
As/(mg/kg) | ≤1.0 | |
Cd/(mg/kg) | ≤0.1 | |
Hg/(mg/kg) | ≤0.1 | |
3-chloro-4,5-dioxo-4,5-dihydro-1H-pyrrole[2,3-f]quinoline-2,7,9-tricarboxylic acid,g/100g | ≤0.8 | |
4-nitro-5-methoxy-1H -pyrrole [2,3-f] quinoline-2,7, 9-tricarboxylic acid,g/100g | ≤0.5 | |
Acceptance date | 08/03/2021, 衛食新申字(2021)第0004號 | |
Issued date | 17/08/2021, CFSA Opened Public Comment for Kanzan flower and Pyrroloquinoline quinone disodium (PQQ) salt | |
Approved date | 01/03/2022, officially approved by Announcement No.1 of 2022 | |
3.3 Chlamydomonas reinhardtii
Name | Chlamydomonas reinhardtii | |
Basic information | Family: Chlamydomonaceae, Genus:Chlamydomonas | |
Brief introduction of production process | It was prepared by Chlamydomonas reinhardtii algae species for breeding, fermenter heterotrophic expansion culture and spray-drying. | |
Quality requirement | Property | Green powder |
Protein content | ≥30.0% | |
Crude polysaccharide content | ≥10.0% | |
Other information | 1.The scope of use does not include infant and young children food. 2. Food safety indicators are implemented in accordance with the relevant regulations of algae and algae products. | |
Acceptance date | 13/09/2021, 衛食新申字(2021)第0011號 | |
Issued date | 08/12/2021, CFSA Opened Public Comment for Chlamydomonas reinhardtii | |
Approved date | 11/05/2022, officially approved by Announcement No.2 of 2022 | |
3.4 Sugarcane Polyphenols
Name | Sugarcane Polyphenols |
Brief introduction of production process | Using sugarcane as raw material, through squeezing, filtering, extracting and removing excess sugar and salt to obtain sugarcane molasses, and then water and ethanol are used as extraction solvents to prepare powdered sugarcane extract through processes such as filtration, vacuum concentration, ion exchange and spray drying; or to prepare liquid extract through processes such as filtration and vacuum distillation. |
Recommended daily intake | ≤1 g/d(powder), or≤10 g/d(liquid) The recommended daily intake of powder with total polyphenol content of 200 g/kg is 1 g/d, and liquid with total polyphenol content of 14.8 g/kg is 10g/d. If the above content is exceeded, it shall be converted according to the actual content. |
Other information | 1. Infants, young children, pregnant women and lactating women are not suitable for consumption. The labels and instructions shall indicate the unsuitable population. 2. See appendix for quality specifications and food safety indicators. |
Acceptance date | 18/05/2021, 衛食新申字(2021)第0008號 |
Issued date | 01/11/2021, CFSA Opened Public Comment for Sugarcane Polyphenols and Bifidobacterium longum subsp. longum BB536 |
Approved date | 11/05/2022, officially approved by Announcement No.2 of 2022 |
3.5 Bifidobacterium longum subsp. longum BB536
Name | Bifidobacterium longum subsp. longum BB536 |
Other information | 1. Can be used in infant food 2. Food safety indicators should conform to relevant Chinese standards |
Acceptance date | 18/05/2021, 衛食新進申字(2021)第0003號 |
Issued date | 01/11/2021, CFSA Opened Public Comment for Sugarcane Polyphenols and Bifidobacterium longum subsp. longum BB536 |
Approved date | 11/05/2022, officially approved by Announcement No.2 of 2022 |
4. Penthorum chinense Pursh., one terminated reviewed product
Penthorum chinense Pursh. was included in the termination review list in the first half of 2022. Details are as follows:
Name | Penthorum chinense Pursh. |
Review Opinions | NHC Announcement No.4 of 2020 has approved this product as a new food raw materials for bubble drink, and this time to extend using scope to beverages. Based on the safety assessment information provided, it was agreed to use for beverages and recommended to terminate the review. In addition to the edible way, it is implemented in accordance with No.4 of 2020. |
Summary
New food raw materials has a very strict review and management mechanism, generally speaking, the application timeline is long. However, it is gratifying that the five products approved this year took only about one year from its acceptance to formal approval. Meanwhile, the acceptance number of new food raw materials has been increasing, which also boost the innovation and upgrading of the food industry.
In short, it is suggested that enterprises should strengthen the understanding of regulations and get well prepared for getting new food raw materials approved.
If you have any needs or questions, please contact us at service@jianzaoshiwang.cn.
Note: There may be omissions and errors in the data statistics process because of the change of the official website. The data in this article is for reference only. Please refer to the official information published by the government.