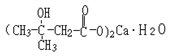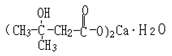On October 21, 2022, China National Center for Food Safety Risk Assessment (CFSA) asked for public comment on Calcium β-hydroxy-β-methyl butyrate (CaHMB). The deadline for public comments is November 21, 2022.
CaHMB was approved as a new food raw material in 2011, and then its scope of application was expanded in 2017. This time, the public consultation plans to expand the recommended intake from ≤3 g/ d to ≤6 g/ day.
Please see the Comparison Table below for more details.
Content of original Notice (2011) | Content of new Notice (2017) | Public Comment Draft (2022) | |
Latin/English name | Calcium β- hydroxy -β- methyl butyrate (CaHMB) | Calcium β-hydroxy -β-methyl butyrate (CaHMB) | Same as the Notice 2017 |
Basic information | Structural formula:
Molecular formula: C10H18O6Ca•H2O Molecular weight: 292 | Structural formula:
Molecular formula: C10H18O6Ca•H2O Molecular weight: 292 | Same as the Notice 2017 |
Production process | With sodium hypochlorite, diacetone alcohol, hydrochloric acid, ethyl acetate, ethanol, calcium hydroxide as the main raw materials, produced by oxidation synthesis, acidification, extraction, neutralization reaction, centrifugation, drying, and other steps. | With sodium hypochlorite, diacetone alcohol, hydrochloric acid, ethyl acetate, ethanol, calcium hydroxide as the main raw materials, produced by oxidation synthesis, acidification, extraction, neutralization reaction, centrifugation, drying, and other steps. | Same as the Notice 2017 |
Recommended intake | ≤3 g/d | ≤3 g/d | ≤6 g/d |
Application scope | Sports nutrition foods, and foods for special medical purposes. | Beverages, milk and dairy products, cocoa products, chocolate and its products, candy, baked foods, sports nutrition foods, and foods for special medical purposes. | Same as the Notice 2017. |
Quality requirement | Property: White powder CaHMB: 77-82% Ca: 12-16% Moisture: 5-7.5% | Property: White powder CaHMB (g/100g): 77-82 Ca (g/100g): 12-16 Moisture (g/100g): 5-7.5 | Please refer to the attachment of the website at the end of this article. |
Other information | Unsuitable group: Infants, children, pregnant women, and breastfeeding women. Labels and instructions should indicate the unsuitable group and the Recommended intake. | 1. Unsuitable group: Infants, children, pregnant women, and breastfeeding women. Labels and instructions should indicate the unsuitable group and the Recommended intake. 2. Hygiene and safety indicators shall conform to the relevant national standards | 1. Unsuitable group: Infants, children, pregnant women, and breastfeeding women. Labels and instructions should indicate the unsuitable group and the Recommended intake. 2. Please refer to the attachment of the website at the end of this article for food safety indicators and quality specifications. |
Notice date | 2011-01-21(2011 Notice No.1) | 2017-06-08 (2018 Notice No.7) | 2022-10-21 (Public Comment Draft) |
If you have any needs or questions, please contact us at service@jianzaoshiwang.cn.
For official info please click:
CFSA Opened Public Comment for Calcium β-hydroxy-β-methyl butyrate