With the release of the Directory of Health Functions Available to Be Claimed for Health Food - Non-nutrition Supplements (2023 Version) (hereinafter referred to as the 2023 Directory) and the supporting documents by the State Administration for Market Regulation (SAMR) on August 31, 2023, the long-awaited change of health food registration certificate policy is finally in place after nearly 20 years. Among the various aspects, the one that attracts the most attention from businesses is the change of certificates for products without validity period and technical requirements.
News Updates
On December 27, 2023, the State Administration for Market Regulation (SMAR) issued the Supporting Documents for the Change of Health Food Registration Certificate for public comments., following the release of the new regulations for health food registration. This document aims to standardize the health food registration certificates with no validity period and technical requirements issued by the former Ministry of Health (MOH). The deadline for public comments is January 26, 2024. More details can be found here.
From 1996 to July 1, 2005, the former Ministry of Health (MOH) and the State Food and Drug Administration approved nearly ten thousand such products. Therefore, CIRS Group collects data and makes a detailed summary of them, aiming to help businesses gain an overview of these products and make early preparations accordingly.
Overview of Health Food Registration from 1996 to 2005
According to statistics, a total of 8345 health foods were registered from 1996 to July 1, 2005, of which 7802 were produced domestically and 543 imported.
Note: Due to the varying number of health functions for such products, multiple health functions in the same approval certificate are counted repeatedly in this article. Those without health functions recorded in the Special Food Information Query Platform are excluded.
Among the products listed in the 2023 Directory, the eight categories of health foods that need to redo or supplement function tests totaled 5385 (5089 domestically produced and 296 imported), accounting for 64.5% of the total approval certificates; and the 16 categories of products that are not required to redo or supplement function tests totaled 1975 (1873 domestically produced and 102 imported), making up 23.7% of the total.
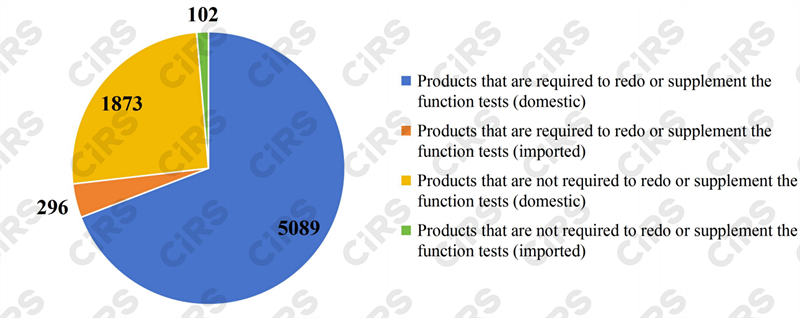
Fig.1 Health foods listed in the 2023 Directory between 1996-2005
Products not listed in the 2023 Directory account for 11.8% of the total approval certificates. Among them, there are 804 products (680 domestically produced and 124 imported) with the health function of supplementing nutrients. In the future, relevant enterprises may complete the change of certificate through filing. Moreover, there are 181 in other categories (160 domestic products and 21 imported products) with functions such as anti-mutagenesis, improving micro-circulation and others that have been removed. Relevant enterprises may apply for new health functions or make adjustments to comply with the 2023 Directory, so as to complete the process.
Health Foods that Are Not Required to Redo or Supplement Function Tests from 1996 to 2005
Among the approval certificates that do not need to redo or supplement functional tests, the largest number is for “Improving gastrointestinal function (promoting regular bowel movements), relieving constipation”, totaling 346, accounting for 17.5% of the sixteen categories of functions, followed by “improving sleep” and “regulate blood sugar levels, assist in lowering blood sugar”, with 256 and 250 approvals respectively, making up 13.0% and 12.7% of the total.

Fig. 2 Number of products that are not required to redo or supplement function tests for each function (1996-2005.7.1)
Among the 1873 domestic approval certificates, the highest number is for “Improving gastrointestinal function (promoting regular bowel movements), relieving constipation”, totaling 326. Following this, there are “Regulate blood sugar, assist in lowering blood sugar” and “Improving sleep” with 250 and 220 approvals respectively. The least applied function is “Improving gastrointestinal function (providing auxiliary protective function for gastric mucosal damage)”, with 27 approvals.
Regarding the 102 imported approvals, the functions with the highest number of approvals are “Improving sleep”, “Improving gastrointestinal function (promoting regular bowel movements)”, and “Soothing and moistening the throat, clearing the throat”, totaling 36, 20, and 11 respectively. Functions include “Improving eyesight, alleviating eye fatigue”, “Improving nutritional anemia, improving iron-deficiency anemia”, “Improving gastrointestinal function (promoting regular bowel movements), relieving constipation”, “Regulating blood sugar levels, assisting in lowering blood sugar” and “Promoting lead excretion” all have zero registered approvals.
Health Foods that Are Required to Redo or Supplement Function Tests from 1996 to 2005
In terms of the analysis by year, the highest number of health food approval certificates requiring a re-do or supplement of function tests is in 2004, totaling 1133, making up 21.0% of the eight functional categories. Following closely is 1997 with 814 approvals, accounting for 15.1%, and 2000 with 587 applications, representing 10.9%.
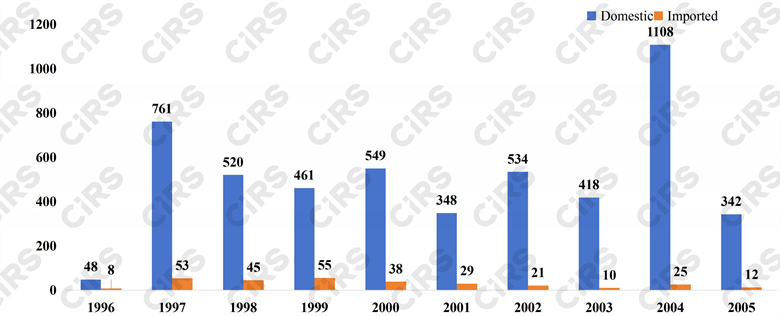
Fig. 3 Number of products that need to redo or supplement function tests in each year
In terms of health functions, “Immunity regulation, enhancing immunity” has the highest count at 2167 among the total approvals, accounting for 40.2% of the eight categories. Following are “Anti-fatigue, alleviating physical fatigue” and “Regulate blood lipid (reduce total cholesterol and lower triglycerides), assisting in lowering blood lipids”, totaling 1081 and 992, making up 20.1% and 18.4% , respectively.

Fig. 4 Number of products that are required to redo or supplement function tests for each function (1996-2005.7.1)
Of the 5089 domestic applications, the top three functions are “Immunity regulation, enhancing immunity”, “Anti-fatigue, alleviating physical fatigue” and “Regulate blood lipid (reduce total cholesterol and lower triglycerides), assisting in lowering blood lipids”, with 2055, 1038, and 882 registrations, respectively.
Among the 296 imported approvals, “Immunity regulation, enhancing immunity”, “Regulate blood lipid (reduce total cholesterol and lower triglycerides), assisting in lowering blood lipids”, and “Anti-fatigue, alleviating physical fatigue” rank the top three functions, with 112, 110, and 43 registrations, respectively. The function “Radiation resistance, providing an auxiliary protective effect against radiation hazards” had no registered application.
CIRS Opinion
Of the 8345 approvals from 1996 to 1 July 2005, 5385 fall under the eight categories of products that need to redo or supplement the function tests, making up 64.5% of the total, among which, products with immunity-related functions hold the highest count, representing 40.2% within the eight functional categories. As stipulated by the policy, function tests need to be redone or supplemented for products evaluated based on the Health Food Function Evaluation Procedures and Testing Methods (1996 Version). However, if a company has already conducted function tests according to the 2003 evaluation method or the 2012 revised nine health function evaluation methods, there is no need to redo the tests.
According to the supporting documents of the 2023 Directory, for products with no validity period and technical requirements, provincial-level market supervision departments should provide opinions on the replacement of registration certificates based on the actual technical requirements of production and regulatory conditions. Therefore, CIRS Group believes that for products that have not been produced or sold after obtaining the approval, the change of certificate process can only commence after actual production and sales.
The Food Division of CIRS Group boasts rich experience in the field of health food registration and filing. We have introduced the change of health food registration certificate service, facilitating enterprises in completing the process within five years in accordance with relevant laws and regulations and ensuring a smooth transition.
If you need any assistance or have any questions, please get in touch with us via service@jianzaoshiwang.cn.
Note:
- Data source: State Administration for Market Regulation Special Food Information Query Platform.
- Statistical scope: Products approved by the former Ministry of Health from 1996 to 2003 and those approved by the former State Food and Drug Administration during the transition period from 2003 to July 1, 2005.
- Due to the varying number of health functions for such products, multiple health functions in the same approval certificate are counted repeatedly in this article. Those without health functions recorded in the Special Food Information Query Platform are excluded.
- As the Special Food Information Query platform is subject to dynamic updates by the authorities, the information in this article is for reference only.
Further Information
Analysis on Health Food and Dietary Supplement Product Registrations in China in 2022

