In accordance with the announcement of the State Administration for Market Regulation (SAMR) regarding the release of Directory of Health Functions Available to Be Claimed by Health Food - Non-nutrition Supplements (2023 Version) and the supporting documents, related registrants must revise the wording of their health claims to align the latest wording and evaluation requirements, when the health functions are listed in the Directory yet the wording of the health claims is outdated (e.g., regulating immunity or improving sleep).
As a result, a great many holders of registration certificates, especially those registered under the previous Ministry of Health (MOH) should redo or supplement the function tests to obtain a new certificate within a 5-year transitional period.
From 1996 to July 1, 2005, the former Ministry of Health (MOH) and the State Food and Drug Administration approved nearly then thousand such products. According to statistics, a total of 8345 health foods were registered from 1996 to July 1, 2005, of which 7802 were produced domestically and 543 imported.
Holders of certificates that shall apply for change of registration
According to the latest regulations, registered products that fall into both of the following categories are required to redo or supplement function tests and apply for change of registration:
- The function evaluation was done based on the Health Food Function Evaluation Procedures and Methods (1996 Version); and
- Product functions fall within the eight health function categories listed in Table 1.
SAMR will issue new registration certificates to the applicants once they have met the requirements.
Table 1. Eight health function categories that require new or supplementary function tests
|
S.N. |
Previous health functions |
New health functions |
Function tests that need to be redone when the evaluation was done based on the Health Food Function Evaluation Procedures and Methods (1996 Version) |
|
1 |
Regulating immunity; enhancing immunity |
Aids in enhancing immunity |
Redo animal function tests |
|
2 |
Delaying aging; anti-oxidation |
Aids in anti-oxidation |
Supplement human feeding trials |
|
3 |
Improving memory; aids in improving memory |
Aids in improving memory |
Redo human feeding trials if the Wechsler memory scale is used for the trials. |
|
4 |
Anti-fatigue; alleviating physical fatigue |
Alleviating physical fatigue |
Redo animal function tests if pole test is used for motor function testing. |
|
5 |
Tolerant to hypoxia; enhancing hypoxia tolerance |
Tolerant to hypoxia |
Redo animal function tests |
|
6 |
Losing weight |
Aids in controlling body fats |
Redo functional tests |
|
7 |
Regulating blood lipids (lowering total cholesterol and reducing triglycerides); assisting in lowering blood lipids |
Aids in maintaining healthy blood lipid (cholesterol/triglyceride) levels |
Redo human feeding trials |
|
8 |
Radiation resistance; providing auxiliary protective function against radiation hazards |
Provides auxiliary protective action against ionizing radiation hazards |
Redo animal function tests |
Dossier Requirements
- For domestic health food
- Health food registration change application form;
- Letter of commitment for authenticity of the submitted materials;
- Copies of the entity registration certificate of the applicant;
- Copies of the registration certificate and its accompanying documents;
- Detailed information on the requested changes, reasons, and legal basis; and
- Relevant supporting documents.
- For imported health food:
In addition to the materials required for domestic health food, the following should also be submitted:
- Qualification documents issued by competent authorities or legal service agencies in the country (or region) of origin confirming the applicant as the overseas health food manufacturer of the products;
- Certifying documents issued by competent authorities or legal service agencies in the country (or region) of origin proving that the health food has been marketed for more than one year, or safety reports on overseas sales and human consumption;
- If products have been approved by the exporting country (region), sales certificate issued by the competent authorities of the exporting countries (regions) shall be provided;
- Original text of health food-associated technical regulations or standards from the country (or region) of origin or international organizations;
- Samples of packaging, labels, and instructions after change;
- For foreign registration applicants who handle registration affairs through a permanent representative office in China, submit both the original Registration Certificate of Foreign Enterprise’s Permanent Representative Office in China and its copies; for foreign registration applicants who delegate registration matters to domestic agencies, provide the original notarized certificate of entrustment and copies of business license of the agencies entrusted; and
- Documents confirming the changes from the government authorities or legal service agency of the country (or region) of origin.
Matters need attention during the change of application
- Applicants must update the product’s labels, instructions, and technical requirements to ensure compliance with the latest regulatory standards;
- The name of the product should align with the Guideline on Health Food Naming (2019 Version) and relevant provisions. If the approved name does not comply with the requirements, an application for renaming should be submitted;
- If health function evaluation tests are required, the applicant must also submit a hygiene test report for the test samples issued by a legally authorized testing institution; if human feeding trials are necessary, ethical review certificates and human consumption evaluation materials shall also be provided; and
- Applicants can submit multiple change requests simultaneously if needed.
Registration Process
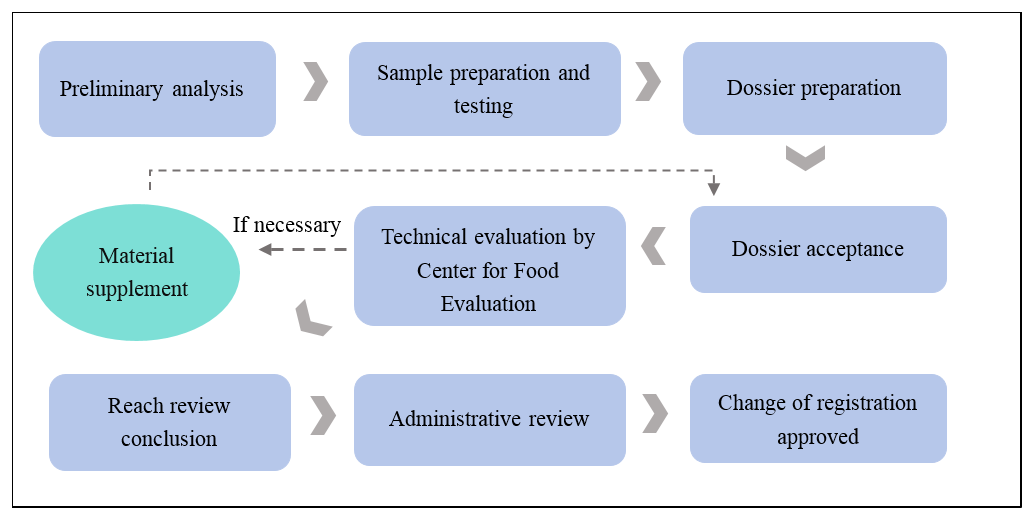
Estimated registration period
Generally, it takes about 1-2 years to complete the change of registration (From dossier preparation, testing to evaluation by CFE).
Our services
- Health Food Formula and Production Process Research and Development
- Domestic/Imported Health Food Registration
- Domestic/Imported Health Food Filing
- Domestic/Imported Health Food Registration Renewal
- Domestic/Imported Health Food Technology Transfer Registration
- New Health Food Functions Application
Further Information
China Health Food Registration and Filing
Registration Status of Health Food in China in the first half of 2023
Filing Status of Chinese Health Foods (Dietary Supplements) in the First Half of 2023