Updated on April 9, 2024
According to the State Administration for Market Regulation (SAMR), the number of foods for special medical purposes (FSMP) approved in China has reached 181. 17 FSMPs have been approved in the first quarter of 2024, including 16 domestic products and 1 imported. These include 3 domestic nutritionally complete products, 8 domestic nutritionally incomplete products, 5 domestic infant formula for special medical purposes, and 1 imported infant formula for special medical purposes. Details are as follows:
Table 1. List of newly approved FSMP products
|
S.N. |
Applicant |
Product name |
Dosage form |
Certificate number |
Expiry date |
|
1 |
Jilin Boya Nutraceuticals Co., Ltd. |
博佳健® nutritionally complete food for special medical purposes |
Powder |
國食注字TY20240001 |
2029.1.5 |
|
2 |
Heilongjiang Feihe Dairy Co., Ltd. |
蓓舒維preterm/low birth weight infant formula for special medical purpose |
Powder |
國食注字TY20240002 |
2029.1.5 |
|
3 |
Heilongjiang Feihe Dairy Co., Ltd. |
蓓舒煥preterm/low birth weight infant formula for special medical purpose |
Powder |
國食注字TY20240003 |
2029.1.5 |
|
4 |
Nantong Richen Bioengineering Co. LTD. |
小佳太®/nutritionally complete food for special medical purposes |
Powder |
國食注字TY20240004 |
2029.1.5 |
|
5 |
Zhejiang Kelubao Food Co., Ltd. |
嘉璐佰infant nutrition supplement for special medical purpose |
Powder |
國食注字TY20240005 |
2029.1.5 |
|
6 |
Wuxi Hengyi Health Technology Co., Ltd. |
恒益舒棠carbohydrate formula for special medical purpose |
Powder |
國食注字TY20240006 |
2029.1.5 |
|
7 |
Shandong Lihao Food for Special Medical Purpose Co., Ltd. |
咪素®/protein formula for special medical purpose |
Powder |
國食注字TY20240007 |
2029.1.5 |
|
8 |
Linyi Shansong Pharmaceutical Co., Ltd. |
怡貫®/nutritionally complete food for special medical purpose |
Powder |
國食注字TY20240008 |
2029.1.5 |
|
9 |
Nutricia Cuijk B.V. |
愛他美親熠infant partially hydrolyzed milk protein formula for special medical purpose |
Powder |
國食注字TY20245001 |
2029.1.5 |
|
10 |
Tibet DUOXIN HEALTH CO., LTD. |
艾諾佳carbohydrate formula for special medical purpose |
Powder |
國食注字TY20240009 |
2029.2.8 |
|
11 |
Jilin Maifu Nutrition Technology co. LTD |
麥孚卡能®carbohydrate formula for special medical purpose |
Liquid |
國食注字TY20240010 |
2029.2.8 |
|
12 |
Jilin Maifu Nutrition Technology co. LTD |
麥孚樂舒®protein formula for special medical purpose |
Liquid |
國食注字TY20240011 |
2029.2.8 |
|
13 |
Hebei Aisheng Technology Co., Ltd |
諾葆暢® Thickening components formula for special medical purpose |
Powder |
國食注字TY20240012 |
2029.2.8 |
|
14 |
Shijiazhuang Junlebao Taihang Diary Group |
恬適康敏infant partially hydrolyzed milk protein formula for special medical purpose |
Powder |
國食注字TY20240013 |
2029.2.8 |
|
15 |
Tibet DUOXIN HEALTH CO., LTD. |
艾諾優liquid formula for special medical purposes |
Powder |
國食注字TY20240014 |
2029.2.8 |
|
16 |
Shijiazhuang Junlebao Taihang Diary Group |
怡安消lactose-free formula food for special medical purpose |
Powder |
國食注字TY20240015 |
2029.2.8 |
|
17 |
GUANGDONG JUNJOY MEDICAL NUTRITION CO., LTD |
君蓓清protein formula for special medical purpose |
Powder |
國食注字TY20240016 |
2029.2.8 |
FSMP registration and approval status
As of April 1, 2024, a total of 181 FSMPs have obtained approval. Of these, 148 are domestically produced, while 33 are imported. Notably, among the 181 approved products, powdered formulations predominate, with 137 being powdered, whereas the remaining 44 are in liquid form.

Analysis of registered FSMP Types
Since the successful registration of the first infant formula for special medical purposes in 2017, the pathway for registering FSMP has officially opened. As shown in Figure 3, it initially saw high registrations followed by a decline, with registrations predominantly concentrated in 2018 and 2019. On the other hand, registrations for nutritionally incomplete formulas were focused in 2021 and 2023. In 2022, although the overall registration was relatively low, there was an increase in the variety of registered products, including the first specific nutritionally complete formula food. In 2023, the first thickening module was introduced, and registrations mainly centered around both nutritionally complete and incomplete products, accounting for as much as 87%. Compared to the registration volume in 2022, there was a significant surge in registrations in 2023. By the first quarter of 2024, 17 new products have been added, indicating a strong momentum, with specific nutritionally complete foods still requiring further breakthroughs.
Detailed FSMP registration types over the years are illustrated in the figure below.
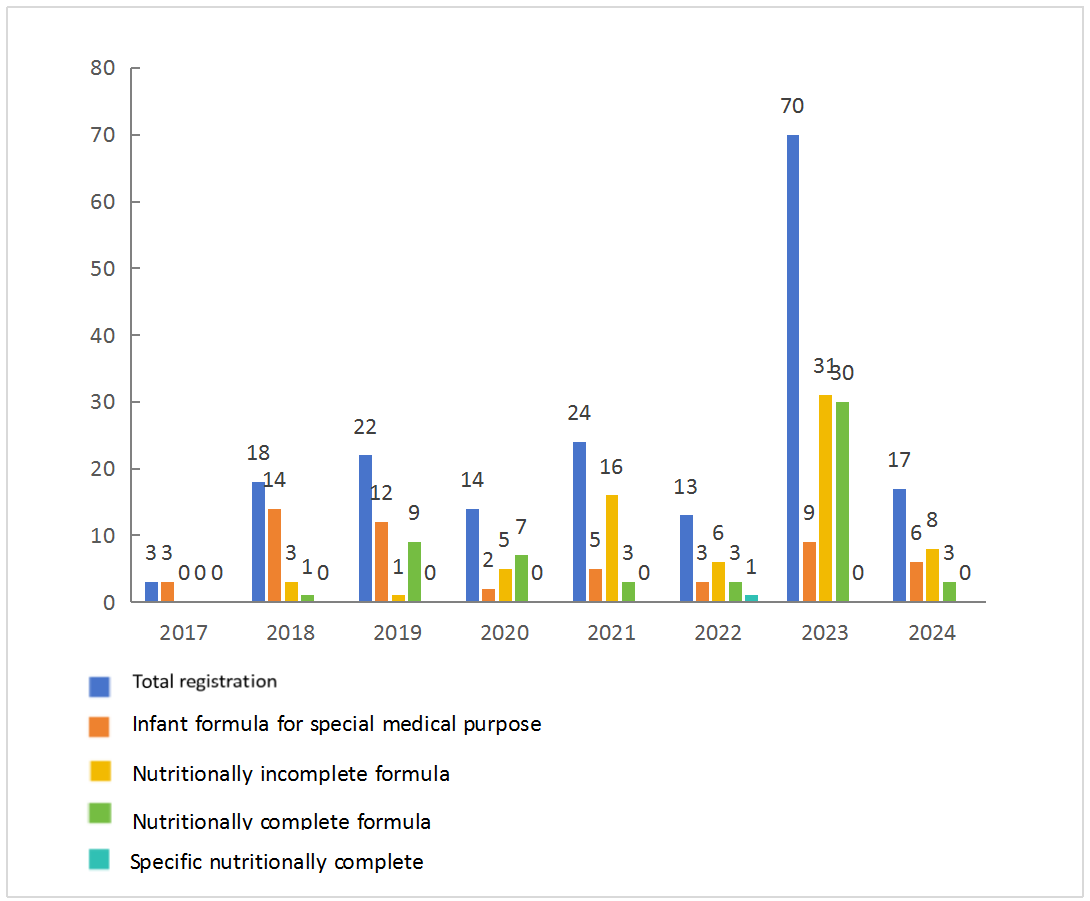
Fig. 3 FSMP registration over the years
Distribution of approved product types
In terms of product types, as shown in the following chart, among the approved products, 54 are infant formula foods for special medical purposes, accounting for 30% of the total. This includes 5 deep hydrolyzed protein or amino acid formulas, 11 partially hydrolyzed protein formulas, 15 lactose-free or low-lactose formulas, 1 amino acid metabolic disorder formula, 17 premature/low birth weight infant formulas, and 5 nutritional supplements. Additionally, there are 70 nutritionally incomplete formula foods, including 21 protein modules, 1 fat module, 18 carbohydrate modules, 23 electrolyte formulas, 2 thickening modules, 3 liquid formulas, and 2 amino acid metabolic disorder formulas (for ages 1 and above), accounting for 39% of the total. Furthermore, there are 56 nutritionally complete formula foods, representing 32% of the total, including 9 and 47 nutritionally complete formula foods for age 1-10, and age 1 and above, respectively. There is only 1 specific nutritionally complete formula food - 速熠素 tumor nutritionally complete formula food special medical purpose.
Note: For detailed information on the names, registering companies, registration numbers, and expiration dates, please click here to check out the latest list of registered FSMP products (official approval information is subject to updates on the official website).
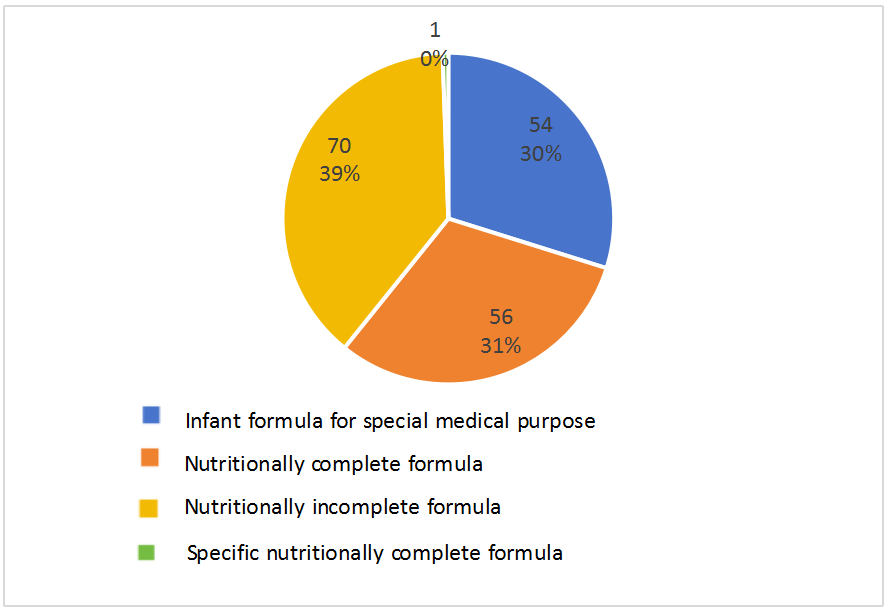
Fig. 4 Distribution of product types
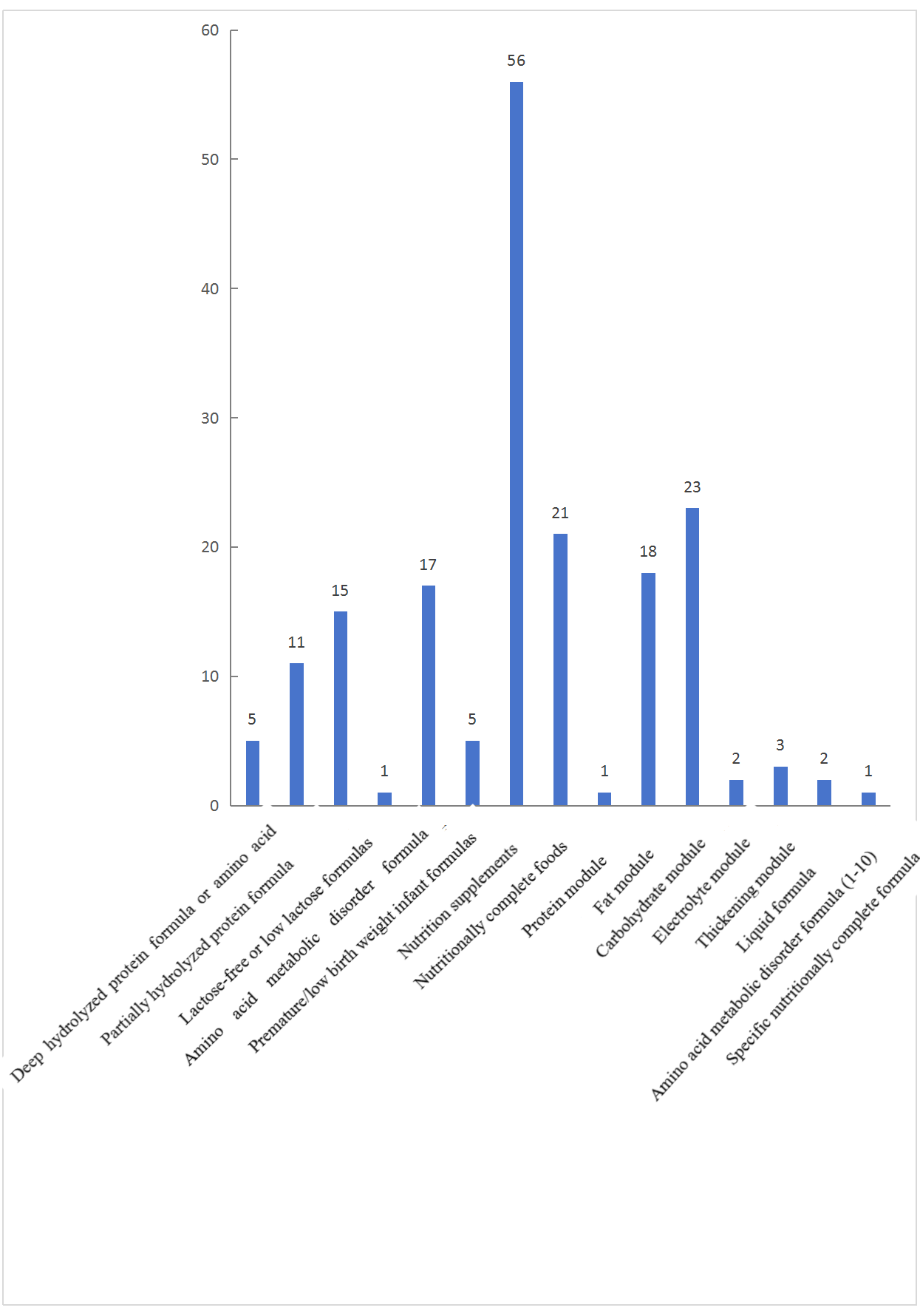
Fig. 5 Registration counts by product category
Details are as follows:
Table 2. FSMP product types and approval status
|
Categories/types |
Approval counts |
Product/Company |
Targeted population |
||
|---|---|---|---|---|---|
|
Infant formula foods for special medical purposes |
Extensively hydrolyzed milk protein formula or amino acid-based formula food |
5 |
Neocate / SHS |
0-12 months |
|
|
ALFARE/ NESTLE |
|||||
|
Althera/NESTLE |
|||||
|
ALFAMINO/ NESTLE |
|||||
|
Pepti Junior/Nutricia |
|||||
|
Partially hydrolyzed milk protein formula food |
11 |
Eleva Care / ABBOTT |
0-12 months |
||
|
Similac / ABBOTT |
|||||
|
EnfaGentlease / MEAD JOHNSON |
|||||
|
優博敏佳/ SYNUTRA |
|||||
|
NAN H.A./Nestle |
|||||
|
舒力樂/ HANGZHOU BEINGMATE |
|||||
|
Similac / ABBOTT |
|||||
|
特愛敏佳/ Qingdao Shengtong |
|||||
|
啟賦®敏適/Nestle |
|||||
|
宜品怡貝/ Yeeper Dairy |
|||||
|
愛他美親熠/ Nutricia |
|||||
|
恬適康敏/Shijiazhuang Junlebao Diary Group |
|||||
|
Lactose free formula or low lactose formula food |
15 |
Beingmate / HANGZHOU BEINGMAT |
0-12 months |
||
|
Enfagrow / MEAD JOHNSON |
|||||
|
安兒寧能恩/ Nestle |
|||||
|
愛思諾賦兒嘉/ Maeil Dairies |
|||||
|
力諾康寧/Tianjin Aumix |
|||||
|
優博瑞安/ SYNUTRA |
|||||
|
葆安素/Yeeper Dairy |
|||||
|
稚舒/ Ausnutria Dairy (China) |
|||||
|
蓓舒消/ Heilongjiang Feihe |
|||||
|
安尼爾/ Wissun International |
|||||
|
愛優諾優安力/ AusNuotore |
|||||
|
特愛瑞安/ Qingdao Shengtong |
|||||
|
嘉復褓/Zhangjiang kelubao |
|||||
|
能盾/ Wissun International |
|||||
|
怡安消/Shijiazhuang Junlebao Diary Group |
|||||
|
Amino acid metabolism disorder formula food |
1 |
Periflex / SHS |
0-12 months |
||
|
Premature or low birth weight infant formula food |
17 |
Similac Neo / ABBOTT |
0-12 months |
||
|
Similac Gro / ABBOTT |
|||||
|
早瑞能恩/ NESTLE |
|||||
|
Enfapro/ MEAD JOHNSON |
|||||
|
優博安能/ SYNUTRA |
|||||
|
Similac/ ABBOTT |
|||||
|
Nutricia/ MILUPA |
|||||
|
早啟能恩/ Nestle |
|||||
|
愛思諾晨而慧/ Maeil Dairies |
|||||
|
貝新爾/ HANGZHOU BEINGMATE |
|||||
|
Wyeth® ULTIMA® 藹而嘉/Wyeth |
|||||
|
甄而藹/ Yeeper Dairy |
|||||
|
愛優諾優健力/ AusNuotore |
|||||
|
特愛安能/ Qingdao Shengtong |
|||||
|
溙敏康/ Zhangjiang Kelubao |
|||||
|
蓓舒維/Heilongjiang Feihe |
|||||
|
蓓舒煥/Heilongjiang Feihe |
|||||
|
Infant nutrition supplement |
5 |
Similac HMFortifi/ ABBOTT |
0-12 months |
||
|
優博啟能/ SYNUTRA |
|||||
|
昔倍護/ HANGZHOU BEINGMATE |
|||||
|
特愛啟能/ Qingdao Shengtong |
|||||
|
嘉璐佰/Zhejiang Kelubao |
|||||
|
FSMP for people over the age of 12 months |
Nutritionally complete foods |
56 |
Pediasure / ABBOTT |
1-10 years old or above 10 |
|
|
佳膳佳立暢/Nestle |
|||||
|
小佰太能/Nestle |
|||||
|
伊利®欣活®/Yili |
|||||
|
ENSURE COMPLETE®/Abbott |
|||||
|
NUTREN JUNIOR /Nestle |
|||||
|
NUTREN® OPTIMUM /Nestle |
|||||
|
優博啟瑞/ SYNUTRA |
|||||
|
力存優太®/Nantong Richen |
|||||
|
麥孚暢清®/Jilin Maifu |
|||||
|
愛優諾優益力/ AusNuotore |
|||||
|
愛優諾優康力/ AusNuotore |
|||||
|
唯卡能®/Harbin Ballansat |
|||||
|
君蓓全/GUANGDONG JUNJOY |
|||||
|
希瑞臻/ SUZHOU HENGRUI JIANKANG |
|||||
|
唯源素®/YABAO |
|||||
|
希瑞怡®/ SUZHOU HENGRUI JIANKANG |
|||||
|
麥孚康全®/ Jilin Maifu |
|||||
|
拜妥優/KWINS HESLTH |
|||||
|
麥孚樂貝®/ Jilin Maifu |
|||||
|
特愛啟瑞/Qingdao Shengtong |
|||||
|
冬澤全太/ DAISY FSMP |
|||||
|
力存全衡素®/Nantong Richen |
|||||
|
麥速®/ Jilin Maifu |
|||||
|
康素得臻膳/ Ausnutria Dairy |
|||||
|
康素得舒膳/ Ausnutria Dairy |
|||||
|
唯源全®/ YABAO |
|||||
|
海維舒®/ Jiangsu Zhengda Fenghai |
|||||
|
海維安®/ Jiangsu Zhengda Fenghai |
|||||
|
卡樂全/ Shandong Ruoyao |
|||||
|
潤能®/ HAISCO PHARMACEUTICAL |
|||||
|
立佳營®/XI'AN LI BANG |
|||||
|
安能®/ HAISCO PHARMACEUTICAL |
|||||
|
麥孚樂寶®/ Jilin Maifu |
|||||
|
立佳泰/ XI'AN LI BANG |
|||||
|
佳安素/ AusNuotore |
|||||
|
江中初元/ Jiangzhong Pharmaceutical |
|||||
|
拜優能/KWINS HEALTH |
|||||
|
辰盈/Cisen Pharmaceutical |
|||||
|
伊盈佳®/Haizheng Sulikang |
|||||
|
能荃力益嘉/NUTRICIA (Wuxi) |
|||||
|
特宜元/SUNMYAN |
|||||
|
小拜妥優/KWINS HEALTH |
|||||
|
恒益啟元/Wuxi Hengyi |
|||||
|
恒益卓元/Wuxi Hengyi |
|||||
|
小佳膳匯立能/Nestle(China) |
|||||
|
冬澤全素®/ DAISY FSMP |
|||||
|
益諾安/ Yeeper Dairy |
|||||
|
雅培®全安素®/ Abbott (Jiaxing) |
|||||
|
他普薈/Zhejiang kelubao |
|||||
|
德瑞怡®/ Fresenius Kabi |
|||||
|
德瑞太®/ Fresenius Kabi |
|||||
|
倍舒然/Heilongjiang Feihe |
|||||
|
博佳健®/Jilin Boya |
|||||
|
小佳太®/ Nantong Richen |
|||||
|
怡貫®/Linyi Shansong |
|||||
|
Specific nutritionally complete foods |
Tumor |
1 |
速熠素/ Nestle |
Above 10 years old |
|
|
Nutritionally incomplete foods |
Electrolyte formula |
23 |
君蓓樂維/Guangdong Junyue |
Above 10 years old |
|
|
冬澤暢康/ DAISY FSMP |
|||||
|
樂賦/ SUZHOU HENGRUI JIANKANG |
|||||
|
特頤欣/ YICHANG HUMANWELL |
|||||
|
葆暢佳/ CSPC Zhongnuo Yaoye |
|||||
|
諾葆素®/Heibei Aisheng |
|||||
|
西泓源®/Jiangshu Xihong |
|||||
|
循康®/ HAISCO PHARMACEUTICAL |
|||||
|
循暢®/ HAISCO PHARMACEUTICAL |
|||||
|
辰樂維®/ Cisen Pharmaceutical |
|||||
|
益護寧/ Zhejiang Yiyuansu |
|||||
|
伊衡佳®/Zhejiang haizheng sulikang |
|||||
|
艾諾利/Tibet DUOXIN |
|||||
|
麥孚樂舒®/Jilin Maifu |
|||||
|
樂棠/ SUZHOU HENGRUI JIANKANG |
Above 18 years old |
||||
|
樞能/ YICHANG HUMANWELL |
|||||
|
若新/ Shandong Ruoyao |
|||||
|
拜瑞恬/ KWINS HEALTH |
|||||
|
恬能®/ Jiangsu Zhengda Fenghai |
10-14 years old |
||||
|
貝耶/ Shandong Ruoyao |
|||||
|
樂潼/ SUZHOU HENGRUI JIANKANG |
1-10 years old |
||||
|
君蓓樂維/GUANGDONG JUNJOY |
Above 1 year old |
||||
|
Nutrient components |
40 |
伊能佳®/ Haizheng Sulikang |
Above 10 years old |
||
|
泉克/ Tibet DUOXIN |
|||||
|
普柔汀®/Guanzhou EnterNutr |
|||||
|
浦索®/ Jilin Maifu |
|||||
|
諾葆平®/ Hebei Aisheng |
|||||
|
葆棠華/ CSPC Zhongnuo Yaoye |
|||||
|
益安喜®/Zhejiang Yiyuansu |
|||||
|
西沁®/Jiangsu Xihong |
|||||
|
冬澤定/ DAISY FSMP |
|||||
|
藍沛/Qingdao Languo |
|||||
|
諾葆安®/ Hebei Aisheng |
|||||
|
唯源泰®/ Yabao Pharmaceutical |
|||||
|
愛優諾優達力/ AusNuotore |
|||||
|
立如箐®/XI'AN LI BANG |
|||||
|
初元安本®/Jiangzhong Pharmaceutical |
|||||
|
嘉膳能/ KWINS HEALTH |
|||||
|
若益/ Shandong Ruoyao |
|||||
|
恒益清元/ Wuxi Hengyi |
|||||
|
愛優諾賦力素/ AusNuotore |
|||||
|
唯卡素®/ Harbin Ballansat |
|||||
|
海能博/ Hangzhou New Quxing |
|||||
|
科惠研/ Nanjing Jade Crane Ming |
|||||
|
冬澤力太/ DAISY FSMP |
|||||
|
若速/ Shandong Ruoyao |
|||||
|
力優宜®/ Nantong Richen |
|||||
|
采衡/Guangdong Yuewei |
|||||
|
伊暢佳®/ Haizheng Sulikang |
|||||
|
恒益舒棠/Wuxi Hengyi |
|||||
|
咪素®/Shandong Lihao |
|||||
|
艾諾佳/Tibet DUOXIN |
|||||
|
君蓓清/Guangdong Junjoy |
|||||
|
君蓓安/ GUANGDONG JUNJOY |
Above 18 years old |
||||
|
富安/Hainan DonglianChangfu |
|||||
|
素乾®/ Jiangsu Zhengda Fenghai |
|||||
|
若和®/Shandong Ruoyao |
|||||
|
冬澤速棠/ DAISY FSMP |
Above 1 year old |
||||
|
欣飴元/ Cisen Pharmaceutical |
|||||
|
冬澤力安/ DAISY FSMP |
|||||
|
冬澤予棠DAISY FSMP |
|||||
|
麥孚卡能®/Jilin Maifu |
|||||
|
Thickening components |
2 |
麥孚順寶® Jilin Maifu |
Above 10 years old |
||
|
諾葆暢®/ Hebei Aisheng |
|||||
|
Liquid formula |
3 |
諾葆舒® /Heibei Aisheng |
Above 10 years old |
||
|
術和®/ Jilin Maifu |
|||||
|
艾諾優/Tibet DUOXIN |
|||||
|
Amino acid metabolism disorder formula |
2 |
Periflex Ad./SHS |
Above 10 years old |
||
|
Periflex®/SHS |
|||||
Analysis of applying companies
In terms of applicants, a total of 52 companies have obtained FSMP registrations. Among these, 44 are domestic companies holding registration certificates. Nestlé continues to lead with the highest number of approved products in China, totaling 15 products, followed by Jilin Maifu. Global industry leaders like Nestlé, Abbott, and Nutricia have secured approvals for products in both non-infant and infant FSMP categories. Meanwhile, domestic companies exhibit a trend towards product specialization. Domestic pharmaceutical companies primarily focus on non-infant FSMP, whereas domestic dairy companies concentrate on infant FSMP. Only a few companies are involved in both infant and non-infant FSMP sectors. In terms of the number of registered companies and products, domestic companies have surpassed foreign counterparts and now dominate the market Currently, FSMP registrations are predominantly held by dairy, nutrition, and pharmaceutical companies.
Details are illustrated in the following chart.
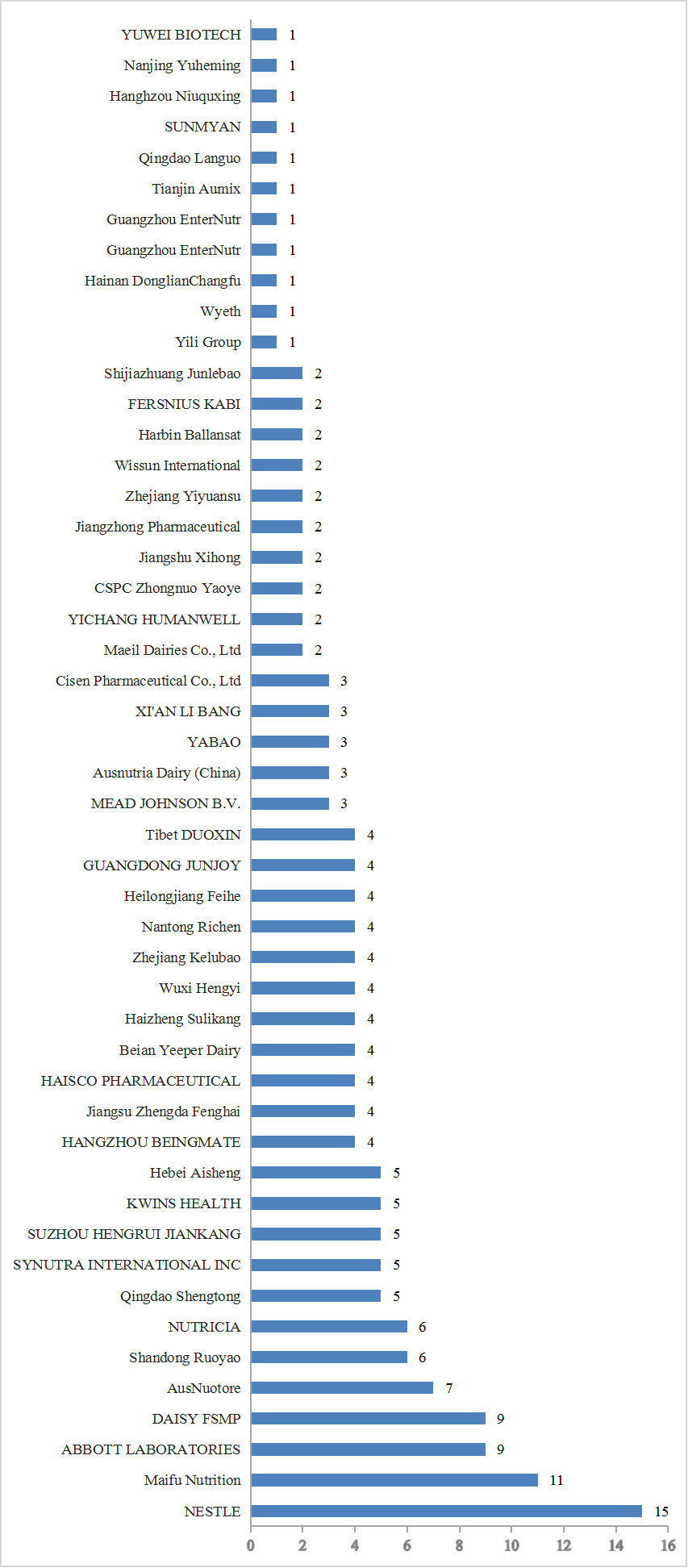
Fig.6 Number of FSMP approved by companies
If you need any assistance or have any questions, please get in touch with us via service@jianzaoshiwang.cn.
Further Information

